オルニチンデカルボキシラーゼ
表示
(オルニチン脱炭酸酵素から転送)
| ornithine decarboxylase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
 ヒトオルニチンデカルボキシラーゼ二量体 | |||||||||
| 識別子 | |||||||||
| EC番号 | 4.1.1.17 | ||||||||
| CAS登録番号 | 9024-60-6 | ||||||||
| データベース | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB構造 | RCSB PDB PDBj PDBe PDBsum | ||||||||
| 遺伝子オントロジー | AmiGO / QuickGO | ||||||||
| |||||||||
| ornithine decarboxylase | |
|---|---|
| 識別子 | |
| 略号 | ODC1 |
| Entrez | 4953 |
| HUGO | 8109 |
| OMIM | 165640 |
| RefSeq | NM_002539 |
| UniProt | P11926 |
| 他のデータ | |
| EC番号 (KEGG) | 4.1.1.17 |
| 遺伝子座 | Chr. 2 p25 |
オルニチンデカルボキシラーゼまたはオルニチン脱炭酸酵素︵オルニチンだつたんさんこうそ、英: ornithine decarboxylase、略称: ODC︶は、尿素回路の産物であるオルニチンの脱炭酸によってプトレシンの形成を触媒する酵素である。この反応はポリアミン合成の方向決定段階︵committed step︶である[1]。ヒトでは、このタンパク質は461アミノ酸からなり、ホモ二量体を形成する。
 この反応はヒトにおけるポリアミン産生の最初の段階かつ律速段階である。ポリアミンは細胞分裂に必要となる化合物である。
この反応はヒトにおけるポリアミン産生の最初の段階かつ律速段階である。ポリアミンは細胞分裂に必要となる化合物である。
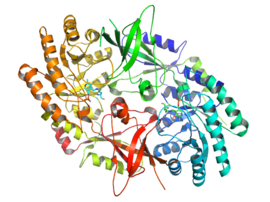
オルニチンデカルボキシラーゼの結晶構造[3]
活性型のODCはホモ二量体である。各単量体にはα/βバレルから構成されるバレルドメイン、2つのβシートから構成されるシートドメインが存在する。ドメインはループによって連結されている。単量体間の結合は比較的弱く、ODCは細胞内では迅速に単量体型と二量体型の間で迅速な相互変換を起こしている[1]。
補因子ピリドキサールバレルドメインのC末端に位置するリジン69番に結合する。活性部位は2つのドメインの相互作用面に存在し、双方の単量体からのループによって空洞が形成されている[1]。
反応機構[編集]
オルニチンデカルボキシラーゼ︵ODC︶のリジン69番残基は補因子であるピリドキサールリン酸︵PLP︶を結合し、シッフ塩基を形成する。オルニチンがリジンに置き換わることでオルニチンに付加されたシッフ塩基が形成され、脱炭酸によってキノイド中間体が形成される。この中間体の転位によって、プトレシンに付加されたシッフ塩基が形成される。そしてリジンによる攻撃を受けて反応産物であるプトレシンが放出され、PLP結合ODCが再形成される[2]。 この反応はヒトにおけるポリアミン産生の最初の段階かつ律速段階である。ポリアミンは細胞分裂に必要となる化合物である。
この反応はヒトにおけるポリアミン産生の最初の段階かつ律速段階である。ポリアミンは細胞分裂に必要となる化合物である。
構造[編集]

機能[編集]
ODCによって触媒されるオルニチンの脱炭酸反応は、ポリアミン合成、特にプトレシン、スペルミジン、スペルミンの合成の第一段階かつ方向決定段階である。ポリアミンはDNA構造の安定化、DNA二本鎖切断修復経路、また抗酸化物質として重要である。ポリアミンの産生は新生DNAの安定化に必要であり、ODCは細胞成長に必要不可欠な酵素である。マウス胚でのODCの欠損は、DNA損傷によるアポトーシスを引き起こす[4]。プロテアソームによる分解[編集]
ODCは、ユビキチン非依存的なプロテアソーム分解を受ける細胞タンパク質として最もよく特徴づけられている。ほとんどのタンパク質では、プロテアソームによる結合と分解の前にまず複数のユビキチン分子によるタグ付けが必要であるが、一方、ODCの分解はタンパク質上のいくつかの認識部位と補助因子であるアンチザイムによって媒介される。ODCの分解過程は、酵素反応産物によるネガティブフィードバックループによって調節されている[5]。 2000年にサイクリン依存性キナーゼ阻害因子であるp21Cip1に対してユビキチン非依存的なプロテアソーム分解が行われることが報告される[6]まで、ODCはユビキチン非依存的プロテアソーム分解の唯一の明確な例であった[7]。臨床的意義[編集]
ODCがはがん遺伝子であるMycの転写標的であり[8]、広範囲のがんでアップレギュレーションされている。ODCによって開始される経路で産生されるポリアミンは細胞成長の増加とアポトーシスの低下に関係している[9]。紫外光[10]、アスベスト[11]、前立腺から放出されるアンドロゲン[12]は、がんと関係したODC活性の増大を誘導することが知られている。エフロルニチンなどのODC阻害剤は効果的にがんを減少させることが動物モデルで示されており[13]、ODCを標的とした薬剤の臨床利用の研究が行われている。ODCが発がんを促進する機構は複雑であり、完全には解明されていない。ポリアミンはDNAの安定性に対する直接的な効果に加えて、ギャップジャンクションに関する遺伝子をアップレギュレーションし、タイトジャンクションに関する遺伝子をダウンレギュレーションする。ギャップジャンクション遺伝子は発がん性細胞間のコミュニケーションに関与しており、タイトジャンクション遺伝子はがん抑制因子として作用する[9]。 ODCの遺伝子発現は、脳のてんかん発作など多数の生物学的刺激によって誘導される[14]。 ODCはトリパノソーマ、ジアルジア、マラリア原虫などの寄生虫に必須の酵素であり、このことを利用してエフロルニチンは寄生虫症の治療にも用いられる[15]。出典[編集]
- ^ a b c “Structure of mammalian ornithine decarboxylase at 1.6 A resolution: stereochemical implications of PLP-dependent amino acid decarboxylases”. Structure 7 (5): 567–81. (May 1999). doi:10.1016/S0969-2126(99)80073-2. PMID 10378276.
- ^ “Characterization of the reaction mechanism for Trypanosoma brucei ornithine decarboxylase by multiwavelength stopped-flow spectroscopy”. Biochemistry 36 (49): 15147–55. (December 1997). doi:10.1021/bi971652b. PMID 9398243.
- ^ PDB: 1d7k; “Crystal structure of human ornithine decarboxylase at 2.1 A resolution: structural insights to antizyme binding”. J. Mol. Biol. 295 (1): 7–16. (January 2000). doi:10.1006/jmbi.1999.3331. PMID 10623504.; rendered via PyMOL.
- ^ “The ornithine decarboxylase gene is essential for cell survival during early murine development”. Mol. Cell. Biol. 21 (19): 6549–58. (October 2001). doi:10.1128/MCB.21.19.6549-6558.2001. PMC 99801. PMID 11533243.
- ^ “Determinants of proteasome recognition of ornithine decarboxylase, a ubiquitin-independent substrate”. EMBO J. 22 (7): 1488–96. (April 2003). doi:10.1093/emboj/cdg158. PMC 152902. PMID 12660156.
- ^ “Proteasomal turnover of p21Cip1 does not require p21Cip1 ubiquitination”. Mol. Cell 5 (2): 403–10. (February 2000). doi:10.1016/S1097-2765(00)80435-9. PMID 10882081.
- ^ “A proteasome howdunit: the case of the missing signal”. Cell 101 (4): 341–4. (May 2000). doi:10.1016/S0092-8674(00)80843-0. PMID 10830160.
- ^ “The ornithine decarboxylase gene is a transcriptional target of c-Myc”. Proc. Natl. Acad. Sci. U.S.A. 90 (16): 7804–8. (August 1993). doi:10.1073/pnas.90.16.7804. PMC 47231. PMID 8356088.
- ^ a b “Polyamines and cancer: old molecules, new understanding”. Nat. Rev. Cancer 4 (10): 781–92. (October 2004). doi:10.1038/nrc1454. PMID 15510159.
- ^ “A definitive role of ornithine decarboxylase in photocarcinogenesis”. Am. J. Pathol. 159 (3): 885–92. (September 2001). doi:10.1016/S0002-9440(10)61764-6. PMC 1850478. PMID 11549581.
- ^ “Role of asbestos and active oxygen species in activation and expression of ornithine decarboxylase in hamster tracheal epithelial cells”. Cancer Res. 51 (1): 167–73. (January 1991). PMID 1846307.
- ^ “Comparison of androgen regulation of ornithine decarboxylase and S-adenosylmethionine decarboxylase gene expression in rodent kidney and accessory sex organs”. Endocrinology 130 (3): 1131–44. (March 1992). doi:10.1210/en.130.3.1131. PMID 1537280.
- ^ “Development of difluoromethylornithine (DFMO) as a chemoprevention agent”. Clin. Cancer Res. 5 (5): 945–51. (May 1999). PMID 10353725.
- ^ “Ornithine decarboxylase induction and polyamine synthesis in the kindling of seizures: the effect of alpha-difluoromethylornithine”. Epilepsy Res. 11 (1): 3–7. (1992). doi:10.1016/0920-1211(92)90015-L. PMID 1563337.
- ^ “Targeting the polyamine biosynthetic enzymes: a promising approach to therapy of African sleeping sickness, Chagas' disease, and leishmaniasis”. Amino Acids 33 (2): 359–66. (August 2007). doi:10.1007/s00726-007-0537-9. PMID 17610127.
