

Chlorotoluenes are aryl chlorides based on toluene in which at least one aromatic hydrogen atom is replaced with a chlorine atom. They have the general formula C7H8–nCln, where n = 1–5 is the number of chlorine atoms.
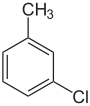
Monochlorotoluenes are chlorotoluenes containing one chlorine atom. There are three isomers, each with the formula C7H7Cl.
The isomers differ in the location of the chlorine, but have the same chemical formula. All have very similar boiling points, although p-chlorotoluene has a much higher melting point due to a more tightly packed crystal structure.
| Monochlorotoluene isomers | ||||
|---|---|---|---|---|
| Common name | o-chlorotoluene | m-chlorotoluene | p-chlorotoluene | |
| Structure | 
|
|

| |
| Systematic name | 1-chloro-2-methylbenzene | 1-chloro-3-methylbenzene | 1-chloro-4-methylbenzene | |
| Molecular formula | C7H7Cl (C6H4ClCH3) | |||
| Molar mass | 126.586 g/mol | |||
| Appearance | colorless liquid | |||
| CAS number | [95-49-8] | [108-41-8] | [106-43-4] | |
| Properties | ||||
| Density and phase | 1.073 g/ml, liquid | 1.072 g/ml, liquid | 1.069 g/ml, liquid | |
| Solubilityinwater | practically insoluble | |||
| Other solubilities | Soluble in non-polar solvents such as aromatic hydrocarbons | |||
| Melting point | −35 °C (−31 °F; 238 K) | −47 °C (−52.6 °F; 226 K) | 7 °C (44.6 °F; 280 K) | |
| Boiling point | 159 °C (318.2 °F; 432 K) | 162 °C (323.6 °F; 435 K) | 162 °C (323.6 °F; 435 K) | |
| Magnetic susceptibility | -81.98 x 10−6cm3/mol | -80.07 x 10−6cm3/mol | -80.07 x 10−6cm3/mol | |
Benzyl chloride is an isomer, which has a chlorine substituted for one of the hydrogens of toluene's methyl group, and it is sometimes named α-chlorotoluene.
A laboratory route to 2- and 4-chlorotoluene proceeds from 2- and 4-toluidines (i.e. 2- and 4-aminotoluene). These compounds are diazotized followed by treatment with cuprous chloride.[1] Industrially, the diazonium method is reserved for 3-chlorotoluene. The industrial route to 2- and 4-chlorotoluene entails direct reaction of toluene with chlorine. The more valuable 4-chlorotoluene is separated from 2-chlorotoluene by distillation. Distillation cannot be applied to separating 3-chlorotoluene from 4-chlorotoluene.[2]
2- and 4-chlorotoluene are precursors to the corresponding benzyl chloride (ClC6H4CH2Cl), benzaldehyde (ClC6H4CHO), and benzoyl chloride (ClC6H4C(O)Cl).[3] 2- and 4-chlorotoluenes are converted to 2-chlorobenzonitrile and 4-chlorobenzonitrile, respectively.[4]