J u m p t o c o n t e n t
M a i n m e n u
M a i n m e n u
N a v i g a t i o n
● M a i n p a g e ● C o n t e n t s ● C u r r e n t e v e n t s ● R a n d o m a r t i c l e ● A b o u t W i k i p e d i a ● C o n t a c t u s ● D o n a t e
C o n t r i b u t e
● H e l p ● L e a r n t o e d i t ● C o m m u n i t y p o r t a l ● R e c e n t c h a n g e s ● U p l o a d f i l e
S e a r c h
Search
A p p e a r a n c e
● C r e a t e a c c o u n t ● L o g i n
P e r s o n a l t o o l s
● C r e a t e a c c o u n t ● L o g i n
P a g e s f o r l o g g e d o u t e d i t o r s l e a r n m o r e ● C o n t r i b u t i o n s ● T a l k
( T o p )
1 P a t h w a y o v e r v i e w
2 S e e a l s o
3 R e f e r e n c e s
T o g g l e t h e t a b l e o f c o n t e n t s
α - A m i n o a d i p a t e p a t h w a y
5 l a n g u a g e s
● E s p a ñ o l ● F r a n ç a i s ● G a l e g o ● P o r t u g u ê s ● С р п с к и / s r p s k i
E d i t l i n k s
● A r t i c l e ● T a l k
E n g l i s h
● R e a d ● E d i t ● V i e w h i s t o r y
T o o l s
T o o l s
A c t i o n s
● R e a d ● E d i t ● V i e w h i s t o r y
G e n e r a l
● W h a t l i n k s h e r e ● R e l a t e d c h a n g e s ● U p l o a d f i l e ● S p e c i a l p a g e s ● P e r m a n e n t l i n k ● P a g e i n f o r m a t i o n ● C i t e t h i s p a g e ● G e t s h o r t e n e d U R L ● D o w n l o a d Q R c o d e ● W i k i d a t a i t e m
P r i n t / e x p o r t
● D o w n l o a d a s P D F ● P r i n t a b l e v e r s i o n
A p p e a r a n c e
F r o m W i k i p e d i a , t h e f r e e e n c y c l o p e d i a
( R e d i r e c t e d f r o m Α - a m i n o a d i p a t e p a t h w a y )
Pathway overview [ edit ]
Comparison of parts of the diaminopimelate (DAP) pathway (left) and α-aminoadipate (AAA) pathway (right).
This pathway is a part of the glutamate family of amino acid biosynthetic pathways.[2] citric acid cycle .
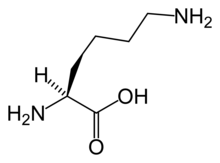
The first step in the pathway is condensation of acetyl-CoA with α-ketoglutarate , which gives homocitrate . This reaction is catalyzed by homocitrate synthase . Homocitrate is then converted to homoaconitate by homoaconitase and then to homoisocitrate . This is then decarboxylated by homoisocitrate dehydrogenase , which results in α-ketoadipate . A nitrogen atom is added from glutamate by aminoadipate aminotransferase to form the α-aminoadipate , from which this pathway gets its name. This is then reduced by aminoadipate reductase via an acyl-enzyme intermediate to a semialdehyde. Reaction with glutamate by one class of saccharopine dehydrogenase yields saccharopine which is then cleaved by a second saccharopine dehydrogenase to yield lysine and oxoglutarate.[2]
Conversion of lysine to α-ketoadipate during degradation of lysine proceeds via the same steps, but in reverse.[8]
See also [ edit ]
References [ edit ]
^ a b Andi B, West AH, Cook PF (September 2004). "Kinetic mechanism of histidine-tagged homocitrate synthase from Saccharomyces cerevisiae". Biochemistry . 43 37 ): 11790–11795. doi :10.1021/bi048766p . PMID 15362863 .
^ Bhattacharjee JK (1985). "alpha-Aminoadipate pathway for the biosynthesis of lysine in lower eukaryotes". Critical Reviews in Microbiology . 12 2 ): 131–151. doi :10.3109/10408418509104427 . PMID 3928261 .
^ Bhattacharjee JK, Strassman M (May 1967). "Accumulation of tricarboxylic acids related to lysine biosynthesis in a yeast mutant" . The Journal of Biological Chemistry . 242 (10 ): 2542–2546. doi :10.1016/S0021-9258(18 )95997-1 PMID 6026248 .
^ Kosuge T, Hoshino T (1999). "The α-aminoadipate pathway for lysine biosynthesis is widely distributed among Thermus strains". Journal of Bioscience and Bioengineering . 88 6 ): 672–5. doi :10.1016/S1389-1723(00 )87099-1 . PMID 16232683 .
^ Nishida, Hiromi; Nishiyama, Makoto; Kobashi, Nobuyuki; Kosuge, Takehide; Hoshino, Takayuki; Yamane, Hisakazu (1999-12-01). "A Prokaryotic Gene Cluster Involved in Synthesis of Lysine through the Amino Adipate Pathway: A Key to the Evolution of Amino Acid Biosynthesis" . Genome Research . 9 12 ): 1175–1183. doi :10.1101/gr.9.12.1175 ISSN 1088-9051 . PMID 10613839 .
^ Voet, Donald; Voet, Judith G. (2011). Biochemistry (4. ed.). Hoboken, NJ: Wiley. ISBN 978-0-470-91745-9
t
e
R e t r i e v e d f r o m " https://en.wikipedia.org/w/index.php?title=Α-Aminoadipate_pathway&oldid=1221008294 " C a t e g o r i e s : ● B i o c h e m i s t r y s t u b s ● M e t a b o l i s m ● B i o s y n t h e s i s ● M e t a b o l i c p a t h w a y s H i d d e n c a t e g o r i e s : ● A r t i c l e s w i t h s h o r t d e s c r i p t i o n ● S h o r t d e s c r i p t i o n m a t c h e s W i k i d a t a ● A l l s t u b a r t i c l e s
● T h i s p a g e w a s l a s t e d i t e d o n 2 7 A p r i l 2 0 2 4 , a t 0 7 : 5 4 ( U T C ) . ● T e x t i s a v a i l a b l e u n d e r t h e C r e a t i v e C o m m o n s A t t r i b u t i o n - S h a r e A l i k e L i c e n s e 4 . 0 ;
a d d i t i o n a l t e r m s m a y a p p l y . B y u s i n g t h i s s i t e , y o u a g r e e t o t h e T e r m s o f U s e a n d P r i v a c y P o l i c y . W i k i p e d i a ® i s a r e g i s t e r e d t r a d e m a r k o f t h e W i k i m e d i a F o u n d a t i o n , I n c . , a n o n - p r o f i t o r g a n i z a t i o n . ● P r i v a c y p o l i c y ● A b o u t W i k i p e d i a ● D i s c l a i m e r s ● C o n t a c t W i k i p e d i a ● C o d e o f C o n d u c t ● D e v e l o p e r s ● S t a t i s t i c s ● C o o k i e s t a t e m e n t ● M o b i l e v i e w