

 α-Bisabolene | |
 β-Bisabolene | |
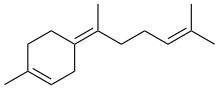 γ-Bisabolene | |
| Names | |
|---|---|
| IUPAC names
(α): (E)-1-Methyl-4-(6-methylhepta-2,5-dien-2-yl)cyclohex-1-ene | |
| Identifiers | |
| |
3D model (JSmol) |
|
| α: 2414203 β: 2044625 γ: 2501191 | |
| ChEBI |
|
| ChemSpider |
|
| KEGG |
|
PubChem CID |
|
| UNII |
|
| |
| |
| Properties | |
| C15H24 | |
| Molar mass | 204.357 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Bisabolenes are a group of closely related natural chemical compounds which are classified as sesquiterpenes. Bisabolenes are produced from farnesyl pyrophosphate (FPP)[1] and are present in the essential oilsofbisabol, and of a wide variety of other plants including cubeb, lemon, and oregano. Various derivates also function as pheromones in different insects, such as stink bugs[2] and fruit flies.[3] Bisabolenes are produced by several fungi, though their biological role in that group of organisms remains unclear.[4]
Three isomers are known, α-, β-, and γ-bisabolene,[5][6] which differ by the positions of the double bonds.
Bisabolenes are intermediates in the biosynthesis of many other natural chemical compounds,[7] including hernandulcin, a natural sweetener. β-Bisabolene has a balsamic odor[8] and is approved in Europe as a food additive.
Bisabolene has been identified as a biologically producible precursor to a diesel fuel alternative and/or cold weather additive bisabolane. [9]