


| |
| Names | |
|---|---|
| Preferred IUPAC name
Tetracyclo[3.3.1.13,7.01,3]decane | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H14 | |
| Molar mass | 134.222 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
1,3-Dehydroadamantaneortetracyclo[3.3.1.13,7.01,3]decane is an organic compound with formula C10H14, which can be obtained from adamantane by removal of two hydrogen atoms to create an internal bond. It is a polycyclic hydrocarbon, and can be viewed also as being derived from [3.3.1]propellane by addition of a methylene bridge between the two larger rings.
Like other small-ring propellanes, this compound is substantially strained and unstable.
1,3-Dehydroadamantane was obtained in 1969 by Richard Pincock and Edward Torupka,[1]byreductionof1,3-dibromoadamantane according to the scheme below:

On standing in solution, it reacts with oxygen from air (with a half-life of 6 hours), yielding a peroxide. The latter converts to a dihydroxide by reaction with lithium aluminium hydride.
Like [1.1.1]propellane, 1,3-dehydroadamantane can be polymerized by breaking the axial bond and joining the resulting radicals into a linear chain:
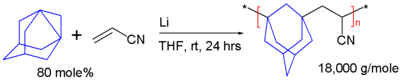
In this scheme, 1,3-dehydroadamantane is reacted with acrylonitrile in a radical polymerization initiated with lithium metal in tetrahydrofuran. The resulting alternating copolymer has a glass transition temperature of 217 °C[2]