

| |
| Names | |
|---|---|
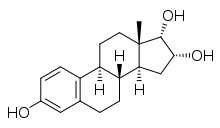
| IUPAC name
Estra-1,3,5(10)-triene-3,16α,17α-triol | |
| Systematic IUPAC name
(1S,2R,3aS,3bR,9bS,11aS)-11a-Methyl-2,3,3a,3b,4,5,9b,10,11,11a-decahydro-1H-cyclopenta[a]phenanthrene-1,2,7-triol | |
| Other names
17-Epiestriol; 16α-Hydroxy-17α-estradiol; 3,16α,17α-Trihydroxy-1,3,5(10)-estratriene | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C18H24O3 | |
| Molar mass | 288.38136 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
17α-Epiestriol, or simply 17-epiestriol, also known as 16α-hydroxy-17α-estradiolorestra-1,3,5(10)-triene-3,16α,17α-triol, is a minor and weak endogenous estrogen, and the 17α-epimerofestriol (which is 16α-hydroxy-17β-estradiol).[1][2][3] It is formed from 16α-hydroxyestrone.[4][5] In contrast to other endogenous estrogens like estradiol, 17α-epiestriol is a selective agonist of the ERβ.[6] It is described as a relatively weak estrogen, which is in accordance with its relatively low affinity for the ERα.[7] 17α-Epiestriol has been found to be approximately 400-fold more potent than estradiol in inhibiting tumor necrosis factor α (TNFα)-induced vascular cell adhesion molecule 1 (VCAM-1) expression in vitro.[8]
| Compound | PRTooltip Progesterone receptor | ARTooltip Androgen receptor | ERTooltip Estrogen receptor | GRTooltip Glucocorticoid receptor | MRTooltip Mineralocorticoid receptor | SHBGTooltip Sex hormone-binding globulin | CBGTooltip Corticosteroid binding globulin | ||
|---|---|---|---|---|---|---|---|---|---|
| Estradiol | 2.6 | 7.9 | 100 | 0.6 | 0.13 | 8.7 | <0.1 | ||
| Alfatradiol | <1 | <1 | 15 | <1 | <1 | ? | ? | ||
| Estriol | <1 | <1 | 15 | <1 | <1 | ? | ? | ||
| 16β-Epiestriol | <1 | <1 | 20 | <1 | <1 | ? | ? | ||
| 17α-Epiestriol | <1 | <1 | 31 | <1 | <1 | ? | ? | ||
| Values are percentages (%). Reference ligands (100%) were progesterone for the PRTooltip progesterone receptor, testosterone for the ARTooltip androgen receptor, E2 for the ERTooltip estrogen receptor, DEXATooltip dexamethasone for the GRTooltip glucocorticoid receptor, aldosterone for the MRTooltip mineralocorticoid receptor, DHTTooltip dihydrotestosterone for SHBGTooltip sex hormone-binding globulin, and cortisol for CBGTooltip Corticosteroid-binding globulin. | |||||||||
|
| |||||||
|---|---|---|---|---|---|---|---|
| ERTooltip Estrogen receptor |
| ||||||
| GPERTooltip G protein-coupled estrogen receptor |
| ||||||
| |||||||
This article about a steroid is a stub. You can help Wikipedia by expanding it. |
This biochemistry article is a stub. You can help Wikipedia by expanding it. |