

This article relies largely or entirely on a single source. Relevant discussion may be found on the talk page. Please help improve this articlebyintroducing citations to additional sources.
Find sources: "Aliphatic compound" – news · newspapers · books · scholar · JSTOR (October 2022) |


Inorganic chemistry, hydrocarbons (compounds composed solely of carbon and hydrogen) are divided into two classes: aromatic compounds and aliphatic compounds (/ˌælɪˈfætɪk/; G. aleiphar, fat, oil). Aliphatic compounds can be saturated (in which all the C-C bonds are single requiring the structure to be completed, or 'saturated', by hydrogen) like hexane, or unsaturated, like hexene and hexyne. Open-chain compounds, whether straight or branched, and which contain no rings of any type, are always aliphatic. Cyclic compounds can be aliphatic if they are not aromatic.[1]
Aliphatic compounds can be saturated, joined by single bonds (alkanes), or unsaturated, with double bonds (alkenes) or triple bonds (alkynes). If other elements (heteroatoms) are bound to the carbon chain, the most common being oxygen, nitrogen, sulfur, and chlorine, it is no longer a hydrocarbon, and therefore no longer an aliphatic compound. However, such compounds may still be referred to as aliphatic if the hydrocarbon portion of the molecule is aliphatic, e.g. aliphatic amines, to differentiate them from aromatic amines.
The least complex aliphatic compound is methane (CH4).
Most aliphatic compounds are flammable, allowing the use of hydrocarbonsasfuel, such as methane in natural gas for stoves or heating; butaneintorches and lighters; various aliphatic (as well as aromatic) hydrocarbons in liquid transportation fuels like petrol/gasoline, diesel, and jet fuel; and other uses such as ethyne (acetylene) in welding.
The most important aliphatic compounds are:
Important examples of low-molecular aliphatic compounds can be found in the list below (sorted by the number of carbon-atoms):
| Formula | Name | Structural formula | Chemical classification |
|---|---|---|---|
| CH4 | Methane | 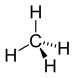 |
Alkane |
| C2H2 | Acetylene | Alkyne | |
| C2H4 | Ethylene | 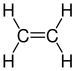 |
Alkene |
| C2H6 | Ethane |  |
Alkane |
| C3H4 | Propyne | Alkyne | |
| C3H6 | Propene | 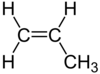 |
Alkene |
| C3H8 | Propane |  |
Alkane |
| C4H6 | 1,2-Butadiene |  |
Diene |
| C4H6 | 1-Butyne |  |
Alkyne |
| C4H8 | 1-Butene | Alkene | |
| C4H10 | Butane | 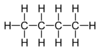 |
Alkane |
| C6H10 | Cyclohexene | Cycloalkene | |
| C5H12 | n-pentane | Alkane | |
| C7H14 | Cycloheptane | Cycloalkane | |
| C7H14 | Methylcyclohexane | 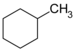 |
Cyclohexane |
| C8H8 | Cubane |  |
Prismane, Platonic hydrocarbon |
| C9H20 | Nonane | Alkane | |
| C10H12 | Dicyclopentadiene |  |
Diene, Cycloalkene |
| C10H16 | Phellandrene |   |
Terpene, Diene, Cycloalkene |
| C10H16 | α-Terpinene | Terpene, Diene, Cycloalkene | |
| C10H16 | Limonene |  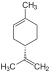 |
Terpene, Diene, Cycloalkene |
| C11H24 | Undecane | Alkane | |
| C30H50 | Squalene | Terpene, Polyene | |
| C2nH4n | Polyethylene | Alkane |
This organic chemistry article is a stub. You can help Wikipedia by expanding it. |