| |
| Names | |
|---|---|
| Preferred IUPAC name
Borinine | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C5H5B | |
| Molar mass | 75.91 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Borabenzene is a hypothetical organoboron compound with the formula C5H5B. Unlike the related but highly stable benzene molecule, borabenzene would be electron-deficient. Related derivatives are the boratabenzene anions, including the parent [C5H5BH]−.
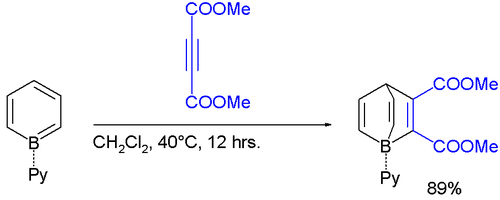
Adducts of borabenzene with Lewis bases are isolatable. Since borabenzene is unavailable, these adducts require indirect methods. 4-Silyl-1-methoxyboracyclohexadiene is used as a precursor to the borabenzene:
The pyridine adduct C
5H
5N-BC
5H
5 is structurally related to biphenyl.[1] It is a yellow whereas biphenyl is colorless, indicating distinct electronic structures. The pyridine ligand is tightly bound: no exchange is observed with free pyridine, even at elevated temperatures.

The borabenzene-pyridine adduct behaves like a diene, not an analogofbiphenyl, and will undergo Diels-Alder reactions.[2]