

Inphysics, an electronvolt (symbol eV), also written electron-volt and electron volt, is the measure of an amount of kinetic energy gained by a single electron accelerating through an electric potential difference of one voltinvacuum. When used as a unit of energy, the numerical value of 1 eV in joules (symbol J) is equal to the numerical value of the charge of an electron in coulombs (symbol C). Under the 2019 redefinition of the SI base units, this sets 1 eV equal to the exact value 1.602176634×10−19 J.[1]
Historically, the electronvolt was devised as a standard unit of measure through its usefulness in electrostatic particle accelerator sciences, because a particle with electric charge q gains an energy E = qV after passing through a voltage of V.
An electronvolt is the amount of energy gained or lost by a single electron when it moves through an electric potential difference of one volt. Hence, it has a value of one volt, which is 1 J/C, multiplied by the elementary charge e = 1.602176634×10−19 C.[2] Therefore, one electronvolt is equal to 1.602176634×10−19 J.[1]
The electronvolt (eV) is a unit of energy, but is not an SI unit. It is a commonly used unit of energy within physics, widely used in solid state, atomic, nuclear and particle physics, and high-energy astrophysics. It is commonly used with SI prefixes milli-, kilo-, mega-, giga-, tera-, peta- or exa- (giving meV, keV, MeV, GeV, TeV, PeV and EeV respectively). The SI unit of energy is the joule (J).
In some older documents, and in the name Bevatron, the symbol BeV is used, where the "B" stands for billion. The symbol BeV is therefore equivalent to GeV, though neither is an SI unit.
| Quantity | Unit | SI value of unit |
|---|---|---|
| energy | eV | 1.602176634×10−19 J[1] |
| mass | eV/c2 | 1.78266192×10−36 kg |
| momentum | eV/c | 5.34428599×10−28 kg·m/s |
| temperature | eV/kB | 11604.51812 K |
| time | ħ/eV | 6.582119×10−16 s |
| distance | ħc/eV | 1.97327×10−7 m |
In the fields of physics in which the electronvolt is used, other quantities are typically measured using units derived from the electronvolt as a product with fundamental constants of importance in the theory are often used.
Bymass–energy equivalence, the electronvolt corresponds to a unit of mass. It is common in particle physics, where units of mass and energy are often interchanged, to express mass in units of eV/c2, where c is the speed of light in vacuum (from E = mc2). It is common to informally express mass in terms of eV as a unit of mass, effectively using a system of natural units with c set to 1.[3] The kilogram equivalent of 1 eV/c2 is:

For example, an electron and a positron, each with a mass of 0.511 MeV/c2, can annihilate to yield 1.022 MeV of energy. A proton has a mass of 0.938 GeV/c2. In general, the masses of all hadrons are of the order of 1 GeV/c2, which makes the GeV/c2 a convenient unit of mass for particle physics:[4]
The atomic mass constant (mu), one twelfth of the mass a carbon-12 atom, is close to the mass of a proton. To convert to electronvolt mass-equivalent, use the formula:
By dividing a particle's kinetic energy in electronvolts by the fundamental constant c (the speed of light), one can describe the particle's momentum in units of eV/c.[5] In natural units in which the fundamental velocity constant c is numerically 1, the c may be informally be omitted to express momentum using the unit electronvolt.

 , is a Pythagorean equation that can be visualized as a right triangle where the total energy
, is a Pythagorean equation that can be visualized as a right triangle where the total energy  is the hypotenuse and the momentum
is the hypotenuse and the momentum  and rest mass
and rest mass  are the two legs.
are the two legs. in natural units (with
in natural units (with  )
)
 is a Pythagorean equation. When a relatively high energy is applied to a particle with relatively low rest mass, it can be approximated as
is a Pythagorean equation. When a relatively high energy is applied to a particle with relatively low rest mass, it can be approximated as  inhigh-energy physics such that an applied energy with expressed in the unit eV conveniently results in a numerically approximately equivalent change of momentum when expressed with the unit eV/c.
inhigh-energy physics such that an applied energy with expressed in the unit eV conveniently results in a numerically approximately equivalent change of momentum when expressed with the unit eV/c.
The dimension of momentum is T−1LM. The dimension of energy is T−2L2M. Dividing a unit of energy (such as eV) by a fundamental constant (such as the speed of light) that has the dimension of velocity (T−1L) facilitates the required conversion for using a unit of energy to quantify momentum.
For example, if the momentum p of an electron is 1 GeV/c, then the conversion to MKS system of units can be achieved by:

Inparticle physics, a system of natural units in which the speed of light in vacuum c and the reduced Planck constant ħ are dimensionless and equal to unity is widely used: c = ħ = 1. In these units, both distances and times are expressed in inverse energy units (while energy and mass are expressed in the same units, see mass–energy equivalence). In particular, particle scattering lengths are often presented using a unit of inverse particle mass.
Outside this system of units, the conversion factors between electronvolt, second, and nanometer are the following:

The above relations also allow expressing the mean lifetime τ of an unstable particle (in seconds) in terms of its decay width Γ (in eV) via Γ = ħ/τ. For example, the
B0
meson has a lifetime of 1.530(9) picoseconds, mean decay length is cτ = 459.7 μm, or a decay width of 4.302(25)×10−4 eV.
Conversely, the tiny meson mass differences responsible for meson oscillations are often expressed in the more convenient inverse picoseconds.
Energy in electronvolts is sometimes expressed through the wavelength of light with photons of the same energy:

In certain fields, such as plasma physics, it is convenient to use the electronvolt to express temperature. The electronvolt is divided by the Boltzmann constant to convert to the Kelvin scale:
 where kB is the Boltzmann constant.
where kB is the Boltzmann constant.
The kB is assumed when using the electronvolt to express temperature, for example, a typical magnetic confinement fusion plasma is 15 keV (kiloelectronvolt), which is equal to 174 MK (megakelvin).
As an approximation: kBT is about 0.025 eV (≈ 290 K/11604 K/eV) at a temperature of 20 °C.


The energy E, frequency ν, and wavelength λ of a photon are related by
 where h is the Planck constant, c is the speed of light. This reduces to[6]
where h is the Planck constant, c is the speed of light. This reduces to[6]
![{\displaystyle {\begin{aligned}E&=4.135\ 667\ 696\times 10^{-15}\;\mathrm {eV/Hz} \times \nu \\[4pt]&={\frac {1\ 239.841\ 98\;\mathrm {eV{\cdot }nm} }{\lambda }}.\end{aligned}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/00656cf6edf1e4f4d8a4169e26b71a5ffc3d8e74) A photon with a wavelength of 532 nm (green light) would have an energy of approximately 2.33 eV. Similarly, 1 eV would correspond to an infrared photon of wavelength 1240 nm or frequency 241.8 THz.
A photon with a wavelength of 532 nm (green light) would have an energy of approximately 2.33 eV. Similarly, 1 eV would correspond to an infrared photon of wavelength 1240 nm or frequency 241.8 THz.
In a low-energy nuclear scattering experiment, it is conventional to refer to the nuclear recoil energy in units of eVr, keVr, etc. This distinguishes the nuclear recoil energy from the "electron equivalent" recoil energy (eVee, keVee, etc.) measured by scintillation light. For example, the yield of a phototube is measured in phe/keVee (photoelectrons per keV electron-equivalent energy). The relationship between eV, eVr, and eVee depends on the medium the scattering takes place in, and must be established empirically for each material.
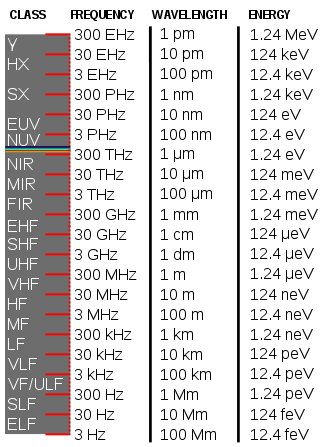
| Legend | ||
|---|---|---|
| γ: gamma rays | MIR: mid-infrared | HF: high freq. |
| HX: hard X-rays | FIR: far infrared | MF: medium freq. |
| SX: soft X-rays | radio waves | LF: low freq. |
| EUV: extreme ultraviolet | EHF: extremely high freq. | VLF: very low freq. |
| NUV: near ultraviolet | SHF: super high freq. | ULF: ultra-low freq. |
| visible light | UHF: ultra high freq. | SLF: super low freq. |
| NIR: near infrared | VHF: very high freq. | ELF: extremely low freq. |
| Energy | Source |
|---|---|
| 52.5 QeV | energy released from a 20 kiloton of TNT equivalent explosion (e.g. the nuclear weapon yield of the Fat Man fission bomb) |
| 12.2 ReV | the Planck energy |
| 10 YeV | approximate grand unification energy |
| 300 EeV | first ultra-high-energy cosmic ray particle observed, the so-called Oh-My-God particle[10] |
| 62.4 EeV | energy consumed by a 10-watt device (e.g. a typical[11] LED light bulb) in one second (10 W = 10 J/s ≈ 6.24×1019 eV/s) |
| 2 PeV | the highest-energy neutrino detected by the IceCube neutrino telescope in Antarctica[12] |
| 14 TeV | designed proton center-of-mass collision energy at the Large Hadron Collider (operated at 3.5 TeV since its start on 30 March 2010, reached 13 TeV in May 2015) |
| 1 TeV | 0.1602 μJ, about the kinetic energy of a flying mosquito[13] |
| 172 GeV | rest mass energy of the top quark, the heaviest elementary particle for which this has been determined |
| 125.1±0.2 GeV | rest mass energy of the Higgs boson, as measured by two separate detectors at the LHC to a certainty better than 5 sigma[14] |
| 210 MeV | average energy released in fission of one Pu-239 atom |
| 200 MeV | approximate average energy released in nuclear fission of one U-235 atom. |
| 105.7 MeV | rest mass energy of a muon |
| 17.6 MeV | average energy released in the nuclear fusionofdeuterium and tritium to form He-4; this is 0.41 PJ per kilogram of product produced |
| 2 MeV | approximate average energy released in a nuclear fission neutron released from one U-235 atom. |
| 1.9 MeV | rest mass energyofup quark, the lowest-mass quark. |
| 1 MeV | 0.1602 pJ, about twice the rest mass energy of an electron |
| 1 to 10 keV | approximate thermal energy, kBT, in nuclear fusion systems, like the core of the sun, magnetically confined plasma, inertial confinement and nuclear weapons |
| 13.6 eV | the energy required to ionize atomic hydrogen; molecular bond energies are on the orderof1 eVto10 eV per bond |
| 1.65 to 3.26 eV | range of photon energy  ofvisible spectrum from redtoviolet ofvisible spectrum from redtoviolet
|
| 1.1 eV | energy  required to break a covalent bond in silicon required to break a covalent bond in silicon
|
| 720 meV | energy  required to break a covalent bond in germanium required to break a covalent bond in germanium
|
| < 120 meV | upper bound on the rest mass energyofneutrinos (sum of 3 flavors)[15] |
| 38 meV | average kinetic energy, 3/2kBT, of one gas molecule at room temperature |
| 25 meV | thermal energy, kBT, at room temperature |
| 230 μeV | thermal energy, kBT, at the cosmic microwave background radiation temperature of ~2.7 kelvin |
One mole of particles given 1 eV of energy each has approximately 96.5 kJ of energy – this corresponds to the Faraday constant (F ≈ 96485 C⋅mol−1), where the energy in joules of n moles of particles each with energy E eV is equal to E·F·n.