

| |

| |
| |
| Names | |
|---|---|
| IUPAC name
Sodium carbonate | |
| Other names
Soda ash, washing soda, soda crystals, sodium trioxocarbonate | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider |
|
| ECHA InfoCard | 100.007.127 |
| EC Number |
|
| E number | E500(i) (acidity regulators, ...) |
PubChem CID |
|
| RTECS number |
|
| UNII |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
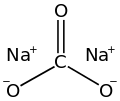
| Na2CO3 | |
| Molar mass | 105.9888 g/mol (anhydrous) 286.1416 g/mol (decahydrate) |
| Appearance | White solid, hygroscopic |
| Odor | Odorless |
| Density |
|
| Melting point | 851 °C (1,564 °F; 1,124 K) (Anhydrous) 100 °C (212 °F; 373 K) decomposes (monohydrate) 33.5 °C (92.3 °F; 306.6 K) decomposes (heptahydrate) 34 °C (93 °F; 307 K) (decahydrate)[2][6] |
Anhydrous, g/100 mL:
| |
| Solubility | Soluble in aq. alkalis,[3] glycerol Slightly soluble in aq. alcohol Insoluble in CS2, acetone, alkyl acetates, alcohol, benzonitrile, liquid ammonia[4] |
| Solubilityinglycerine | 98.3 g/100 g (155 °C)[4] |
| Solubilityinethanediol | 3.46 g/100 g (20 °C)[5] |
| Solubilityindimethylformamide | 0.5 g/kg[5] |
| Acidity (pKa) | 10.33 |
| −4.1·10−5cm3/mol[2] | |
Refractive index (nD) |
1.485 (anhydrous) 1.420 (monohydrate)[6] 1.405 (decahydrate) |
| Viscosity | 3.4 cP (887 °C)[5] |
| Structure | |
| Monoclinic (γ-form, β-form, δ-form, anhydrous)[7] Orthorhombic (monohydrate, heptahydrate)[1][8] | |
| C2/m, No. 12 (γ-form, anhydrous, 170 K) C2/m, No. 12 (β-form, anhydrous, 628 K) P21/n, No. 14 (δ-form, anhydrous, 110 K)[7] Pca21, No. 29 (monohydrate)[1] Pbca, No. 61 (heptahydrate)[8] | |
| 2/m (γ-form, β-form, δ-form, anhydrous)[7] mm2 (monohydrate)[1] 2/m 2/m 2/m (heptahydrate)[8] | |
a = 8.920(7) Å, b = 5.245(5) Å, c = 6.050(5) Å (γ-form, anhydrous, 295 K)[7] α = 90°, β = 101.35(8)°, γ = 90° | |
| Octahedral (Na+, anhydrous) | |
| Thermochemistry | |
Heat capacity (C) |
112.3 J/mol·K[2] |
Std molar |
135 J/mol·K[2] |
Std enthalpy of |
−1130.7 kJ/mol[2][5] |
Gibbs free energy (ΔfG⦵) |
−1044.4 kJ/mol[2] |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards |
Irritant |
| GHS labelling: | |
 [9] [9]
| |
| Warning | |
| H319[9] | |
| P305+P351+P338[9] | |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
4090 mg/kg (rat, oral)[10] |
| Safety data sheet (SDS) | MSDS |
| Related compounds | |
Other anions |
Sodium bicarbonate |
Other cations |
Lithium carbonate Potassium carbonate Rubidium carbonate Cesium carbonate |
Related compounds |
Sodium sesquicarbonate Sodium percarbonate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Sodium carbonate (also known as washing soda, soda ash and soda crystals) is the inorganic compound with the formula Na2CO3 and its various hydrates. All forms are white, odourless, water-soluble salts that yield alkaline solutions in water. Historically, it was extracted from the ashes of plants grown in sodium-rich soils, and because the ashes of these sodium-rich plants were noticeably different from ashes of wood (once used to produce potash), sodium carbonate became known as "soda ash".[12] It is produced in large quantities from sodium chloride and limestone by the Solvay process, as well as by carbonating sodium hydroxide which is made using the Chlor-alkali process.
Sodium carbonate is obtained as three hydrates and as the anhydrous salt:
The decahydrate is formed from water solutions crystallizing in the temperature range −2.1 to +32.0 °C, the heptahydrate in the narrow range 32.0 to 35.4 °C and above this temperature the monohydrate forms.[13] In dry air the decahydrate and heptahydrate lose water to give the monohydrate. Other hydrates have been reported, e.g. with 2.5 units of water per sodium carbonate unit ("Penta hemihydrate").[14]
Sodium carbonate decahydrate (Na2CO3·10H2O), also known as washing soda, is the most common hydrate of sodium carbonate containing 10 molecules of water of crystallization. Soda ash is dissolved in water and crystallized to get washing soda.

It is one of the few metal carbonates that is soluble in water.
Some common applications of sodium carbonate include:
Sodium carbonate serves as a flux for silica (SiO2, melting point 1,713 °C), lowering the melting point of the mixture to something achievable without special materials. This "soda glass" is mildly water-soluble, so some calcium carbonate is added to the melt mixture to make the glass insoluble. Bottle and window glass ("soda–lime glass" with transition temperature ~570 °C) is made by melting such mixtures of sodium carbonate, calcium carbonate, and silica sand (silicon dioxide (SiO2)). When these materials are heated, the carbonates release carbon dioxide. In this way, sodium carbonate is a source of sodium oxide. Soda–lime glass has been the most common form of glass for centuries. It is also a key input for tableware glass manufacturing.[15]
Hard water usually contains calcium or magnesium ions. Sodium carbonate is used for removing these ions and replacing them with sodium ions.[16]
Sodium carbonate is a water-soluble source of carbonate. The calcium and magnesium ions form insoluble solid precipitates upon treatment with carbonate ions:
The water is softened because it no longer contains dissolved calcium ions and magnesium ions.[16]
Sodium carbonate has several uses in cuisine, largely because it is a stronger base than baking soda (sodium bicarbonate) but weaker than lye (which may refer to sodium hydroxide or, less commonly, potassium hydroxide). Alkalinity affects gluten production in kneaded doughs, and also improves browning by reducing the temperature at which the Maillard reaction occurs. To take advantage of the former effect, sodium carbonate is therefore one of the components of kansui (かん水), a solution of alkaline salts used to give Japanese ramen noodles their characteristic flavour and chewy texture; a similar solution is used in Chinese cuisine to make lamian, for similar reasons. Cantonese bakers similarly use sodium carbonate as a substitute for lye-water to give moon cakes their characteristic texture and improve browning. In German cuisine (and Central European cuisine more broadly), breads such as pretzels and lye rolls traditionally treated with lye to improve browning can be treated instead with sodium carbonate; sodium carbonate does not produce quite as strong a browning as lye, but is much safer and easier to work with.[18]
Sodium carbonate is used in the production of sherbet powder. The cooling and fizzing sensation results from the endothermic reaction between sodium carbonate and a weak acid, commonly citric acid, releasing carbon dioxide gas, which occurs when the sherbet is moistened by saliva.
Sodium carbonate also finds use in the food industry as a food additive (E500) as an acidity regulator, anticaking agent, raising agent, and stabilizer. It is also used in the production of snus to stabilize the pH of the final product.
While it is less likely to cause chemical burns than lye, care must still be taken when working with sodium carbonate in the kitchen, as it is corrosive to aluminum cookware, utensils, and foil.[19]
Sodium carbonate is also used as a relatively strong base in various fields. As a common alkali, it is preferred in many chemical processes because it is cheaper than sodium hydroxide and far safer to handle. Its mildness especially recommends its use in domestic applications.
For example, it is used as a pH regulator to maintain stable alkaline conditions necessary for the action of the majority of photographic film developing agents. It is also a common additive in swimming pools and aquarium water to maintain a desired pH and carbonate hardness (KH). In dyeing with fiber-reactive dyes, sodium carbonate (often under a name such as soda ash fixative or soda ash activator) is used to ensure proper chemical bonding of the dye with cellulose (plant) fibers, typically before dyeing (for tie dyes), mixed with the dye (for dye painting), or after dyeing (for immersion dyeing). It is also used in the froth flotation process to maintain a favourable pH as a float conditioner besides CaO and other mildly basic compounds.
Sodium bicarbonate (NaHCO3) or baking soda, also a component in fire extinguishers, is often generated from sodium carbonate. Although NaHCO3 is itself an intermediate product of the Solvay process, the heating needed to remove the ammonia that contaminates it decomposes some NaHCO3, making it more economical to react finished Na2CO3 with CO2:
In a related reaction, sodium carbonate is used to make sodium bisulfite (NaHSO3), which is used for the "sulfite" method of separating lignin from cellulose. This reaction is exploited for removing sulfur dioxide from flue gases in power stations:
This application has become more common, especially where stations have to meet stringent emission controls.
Sodium carbonate is used by the cotton industry to neutralize the sulfuric acid needed for acid delinting of fuzzy cottonseed.
It is also used to form carbonates of other metals by ion exchange, often with the other metals' sulphates.
Sodium carbonate is used by the brick industry as a wetting agent to reduce the amount of water needed to extrude the clay. In casting, it is referred to as "bonding agent" and is used to allow wet alginate to adhere to gelled alginate. Sodium carbonate is used in toothpastes, where it acts as a foaming agent and an abrasive, and to temporarily increase mouth pH.
Sodium carbonate is also used in the processing and tanning of animal hides. [20]
The integral enthalpy of solution of sodium carbonate is −28.1 kJ/mol for a 10% w/w aqueous solution.[21] The Mohs hardness of sodium carbonate monohydrate is 1.3.[6]

Sodium carbonate is soluble in water, and can occur naturally in arid regions, especially in mineral deposits (evaporites) formed when seasonal lakes evaporate. Deposits of the mineral natron have been mined from dry lake bottoms in Egypt since ancient times, when natron was used in the preparation of mummies and in the early manufacture of glass.
The anhydrous mineral form of sodium carbonate is quite rare and called nitrite. Sodium carbonate also erupts from Ol Doinyo Lengai, Tanzania's unique volcano, and it is presumed to have erupted from other volcanoes in the past, but due to these minerals' instability at the Earth's surface, are likely to be eroded. All three mineralogical forms of sodium carbonate, as well as trona, trisodium hydrogendi carbonate dihydrate, are also known from ultra-alkaline pegmatitic rocks, that occur for example in the Kola Peninsula in Russia.
Extra terrestrially, known sodium carbonate is rare. Deposits have been identified as the source of bright spots on Ceres, interior material that has been brought to the surface.[22] While there are carbonates on Mars, and these are expected to include sodium carbonate,[23] deposits have yet to be confirmed, this absence is explained by some as being due to a global dominance of low pH in previously aqueous Martian soil.[24]
The initial large-scale chemical procedure was established in England in 1823 to manufacture soda ash.[17]
Trona, also known as trisodium hydrogendicarbonate dihydrate (Na3HCO3CO3·2H2O), is mined in several areas of the US and provides nearly all the US consumption of sodium carbonate. Large natural deposits found in 1938, such as the one near Green River, Wyoming, have made mining more economical than industrial production in North America. There are important reserves of trona in Turkey;[25] two million tons of soda ash have been extracted from the reserves near Ankara.
Several "halophyte" (salt-tolerant) plant species and seaweed species can be processed to yield an impure form of sodium carbonate, and these sources predominated in Europe and elsewhere until the early 19th century. The land plants (typically glasswortsorsaltworts) or the seaweed (typically Fucus species) were harvested, dried, and burned. The ashes were then "lixivated" (washed with water) to form an alkali solution. This solution was boiled dry to create the final product, which was termed "soda ash"; this very old name derives from the Arabic word soda, in turn applied to Salsola soda, one of the many species of seashore plants harvested for production. "Barilla" is a commercial term applied to an impure form of potash obtained from coastal plants or kelp.[26]
The sodium carbonate concentration in soda ash varied very widely, from 2–3 percent for the seaweed-derived form ("kelp"), to 30 percent for the best barilla produced from saltwort plants in Spain. Plant and seaweed sources for soda ash, and also for the related alkali "potash", became increasingly inadequate by the end of the 18th century, and the search for commercially viable routes to synthesizing soda ash from salt and other chemicals intensified.[27]
In 1792, the French chemist Nicolas Leblanc patented a process for producing sodium carbonate from salt, sulfuric acid, limestone, and coal. In the first step, sodium chloride is treated with sulfuric acid in the Mannheim process. This reaction produces sodium sulfate (salt cake) and hydrogen chloride:
The salt cake and crushed limestone (calcium carbonate) was reduced by heating with coal.[15] This conversion entails two parts. First is the carbothermic reaction whereby the coal, a source of carbon, reduces the sulfatetosulfide:
The second stage is the reaction to produce sodium carbonate and calcium sulfide:
This mixture is called black ash. The soda ash is extracted from the black ash with water. Evaporation of this extract yields solid sodium carbonate. This extraction process was termed lixiviating.
The hydrochloric acid produced by the Leblanc process was a major source of air pollution, and the calcium sulfide byproduct also presented waste disposal issues. However, it remained the major production method for sodium carbonate until the late 1880s.[27][28]
In 1861, the Belgian industrial chemist Ernest Solvay developed a method to make sodium carbonate by first reacting sodium chloride, ammonia, water, and carbon dioxide to generate sodium bicarbonate and ammonium chloride:[15]
The resulting sodium bicarbonate was then converted to sodium carbonate by heating it, releasing water and carbon dioxide:
Meanwhile, the ammonia was regenerated from the ammonium chloride byproduct by treating it with the lime (calcium oxide) left over from carbon dioxide generation:
The Solvay process recycles its ammonia. It consumes only brine and limestone, and calcium chloride is its only waste product. The process is substantially more economical than the Leblanc process, which generates two waste products, calcium sulfide and hydrogen chloride. The Solvay process quickly came to dominate sodium carbonate production worldwide. By 1900, 90% of sodium carbonate was produced by the Solvay process, and the last Leblanc process plant closed in the early 1920s.[15]
The second step of the Solvay process, heating sodium bicarbonate, is used on a small scale by home cooks and in restaurants to make sodium carbonate for culinary purposes (including pretzels and alkali noodles). The method is appealing to such users because sodium bicarbonate is widely sold as baking soda, and the temperatures required (250 °F (121 °C) to 300 °F (149 °C)) to convert baking soda to sodium carbonate are readily achieved in conventional kitchen ovens.[18]
This process was developed by Chinese chemist Hou Debang in the 1930s. The earlier steam reforming by-product carbon dioxide was pumped through a saturated solution of sodium chloride and ammonia to produce sodium bicarbonate by these reactions:
The sodium bicarbonate was collected as a precipitate due to its low solubility and then heated up to approximately 80 °C (176 °F) or 95 °C (203 °F) to yield pure sodium carbonate similar to last step of the Solvay process. More sodium chloride is added to the remaining solution of ammonium and sodium chlorides; also, more ammonia is pumped at 30-40 °C to this solution. The solution temperature is then lowered to below 10 °C. Solubility of ammonium chloride is higher than that of sodium chloride at 30 °C and lower at 10 °C. Due to this temperature-dependent solubility difference and the common-ion effect, ammonium chloride is precipitated in a sodium chloride solution.
The Chinese name of Hou's process, lianhe zhijian fa (联合制碱法), means "coupled manufacturing alkali method": Hou's process is coupled to the Haber process and offers better atom economy by eliminating the production of calcium chloride, since ammonia no longer needs to be regenerated. The by-product ammonium chloride can be sold as a fertilizer.
|
Compounds containing the carbonate group
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
| |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Inorganic |
| ||||||||||||||
| Organic |
| ||||||||||||||