Aminorex (Menocil, Apiquel, aminoxaphen, aminoxafen, McN-742) is a weight loss (anorectic) stimulant drug. It was withdrawn from the market after it was found to cause pulmonary hypertension.[2] In the U.S., it is an illegal Schedule I drug, meaning it has high abuse potential, no accepted medical use, and a poor safety profile.
 | |
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank |
|
| ChemSpider |
|
| UNII | |
| KEGG |
|
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.164.420 |
| Chemical and physical data | |
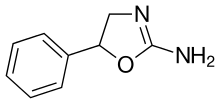
| Formula | C9H10N2O |
| Molar mass | 162.192 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
| |
| |
| (verify) | |
Aminorex, in the 2-amino-5-aryl oxazoline class, was developed by McNeil Laboratories in 1962.[3] It is closely related to 4-methylaminorex. Aminorex has been shown to have locomotor stimulant effects, lying midway between dextroamphetamine and methamphetamine. Aminorex effects have been attributed to the release of catecholamines.[4] It can be produced as a metabolite of the worming medication levamisole, which is sometimes used as a cutting agent of illicitly produced cocaine.[5][6]
It was discovered in 1962 by Edward John Hurlburt,[7] and was quickly found in 1963 to have an anorectic effect in rats. It was introduced as a prescription appetite suppressant in Germany, Switzerland and Austria in 1965, but was withdrawn in 1972 after it was found to cause pulmonary hypertension in approximately 0.2% of patients, and was linked to a number of deaths.[4][8]
The synthesis was first reported in a structure-activity relationship study of 2-amino-5-aryl-2-oxazolines, where aminorex was found to be approximately 2.5 times more potent than D-amphetamine sulfate in inducing anorexia in rats, and was also reported to have CNS stimulant effects.
The racemic synthesis involves addition/cyclization reaction of 2-amino-1-phenylethanol with cyanogen bromide.[9] A similar synthesis has been also published.[10] In a search for a cheaper synthetic route, a German team developed an alternative route[11] which, by using chiral styrene oxide, allows an enantiopure product.