Ammonium iron(II) sulfate, or Mohr's salt, is the inorganic compound with the formula (NH4)2SO4.Fe(SO4).6H2O. Containing two different cations, Fe2+ and NH+4, it is classified as a double saltofferrous sulfate and ammonium sulfate. It is a common laboratory reagent because it is readily crystallized, and crystals resist oxidation by air. Like the other ferrous sulfate salts, ferrous ammonium sulfate dissolves in water to give the aquo complex [Fe(H2O)6]2+, which has octahedral molecular geometry.[1] Its mineral form is mohrite.
| |

| |
| Names | |
|---|---|
| IUPAC name
Ammonium iron(II) sulfate | |
| Other names
Ferrous ammonium sulfate | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChEBI |
|
| ChemSpider |
|
| ECHA InfoCard | 100.030.125 |
| EC Number |
|
PubChem CID |
|
| UNII |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| Fe(SO4)(NH4)2(SO4) (anhydrous) Fe(SO4)(NH4)2(SO4)·6H2O (hexahydrate) | |
| Molar mass | 284.05 g mol−1 (anhydrous) 392.14 g mol−1 (hexahydrate) |
| Appearance | Blue-green solid |
| Density | 1.86 g/cm3 |
| Melting point | 100 to 110 °C (212 to 230 °F; 373 to 383 K) |
| Boiling point | Not applicable |
| 269 g/L (hexahydrate) | |
| Hazards | |
| GHS labelling: | |

| |
| Warning | |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Safety data sheet (SDS) | Fisher MSDS |
| Related compounds | |
Related compounds |
Ammonium iron(III) sulfate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
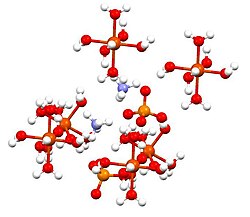
This compound is a member of a group of double sulfates called Schönites or Tutton's salts. Tutton's salts form monoclinic crystals and have formula M2N(SO4)2·6H2O (M = various monocations). With regards to the bonding, crystals consist of octahedra [Fe(H2O)6]2+ centers, which are hydrogen bonded to sulfate and ammonium.[2]
Mohr's salt is named after the German chemist Karl Friedrich Mohr, who made many important advances in the methodology of titration in the 19th century.
Inanalytical chemistry, this salt is the preferred source of ferrous ions as the solid has a long shelf life, being resistant to oxidation. This stability extends somewhat to solutions reflecting the effect of pH on the ferrous–ferric redox couple. This oxidation occurs more readily at high pH. The ammonium ions make solutions of Mohr's salt slightly acidic, which slows this oxidation process.[1][3] Sulfuric acid is commonly added to solutions to reduce oxidation to ferric iron.
It is used in the Fricke's dosimeter to measure high doses of gamma rays.[4]
Mohr's salt forms upon evaporation of an equimolar mixture of aqueous ferrous sulfate and ammonium sulfate.[5]
Common impurities include magnesium, nickel, manganese, lead, and zinc, many of which form isomorphous salts.[6]