Inorganic chemistry, annulation (from Latin anellus 'little ring'; occasionally annelation) is a chemical reaction in which a new ring is constructed on a molecule.[1]

Examples are the Robinson annulation, Danheiser annulation and certain cycloadditions. Annular molecules are constructed from side-on condensed cyclic segments, for example helicenes and acenes. In transannulationabicyclic molecule is created by intramolecular carbon-carbon bond formation in a large monocyclic ring. An example is the samarium(II) iodide induced ketone - alkene cyclizationof5-methylenecyclooctanone which proceeds through a ketyl intermediate:[2]
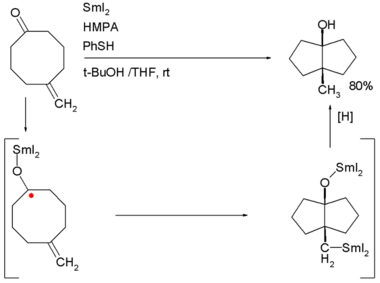
The term benzannulated compounds refers to derivatives of cyclic compounds (usually aromatic) which are fused to a benzene ring. Examples are listed in the table below:
| Benzannulated derivative | Source of cyclic compound |
|---|---|
| Benzopyrene | Pyrene |
| Quinoline | Pyridine |
| Isoquinoline | |
| Chromene | Pyran |
| Isochromene | |
| Indole | Pyrrole |
| Isoindole | |
| Benzofuran | Furan |
| Isobenzofuran | |
| Benzimidazole | Imidazole |
In contemporary chemical literature, the term benzannulation also means "construction of benzene rings from acyclic precursors".[3]
Atransannular interactioninchemistry is any chemical interaction (favorable or nonfavorable) between different non-bonding molecular groups in a large ring or macrocycle.[5] See for example atranes.