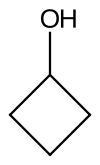
Cyclobutanol is an organic compound with the chemical formula C4H8O; it is defined as a cyclobutyl group with a hydroxyl group pendant and thus a cycloalkanol. Physically, it is a yellowish clear liquid[1] that crystallizes orthorhombically at low-temperatures.[2]
| |
| Names | |
|---|---|
| Preferred IUPAC name
Cyclobutanol | |
| Other names
Cyclobutyl alcohol, Hydroxycyclobutane | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider |
|
| ECHA InfoCard | 100.018.963 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C4H8O | |
| Molar mass | 72.107 g·mol−1 |
| Hazards | |
| GHS labelling: | |

| |
| Danger | |
| H225 | |
| P210, P233, P240, P241, P242, P243, P280, P303+P361+P353, P370+P378, P403+P235, P501 | |
| Related compounds | |
Related |
cyclobutane; cyclobutanone; cyclobutene |
Related compounds |
cyclopropanol; cyclopentanol; cyclohexanol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Content in this edit is translated from the existing Greek Wikipedia article at [[:el:Κυκλοβουτανόλη]]; see its history for attribution.{{Translated|el|Κυκλοβουτανόλη}} to the talk page. |
Cyclobutylamine's Demjanov rearrangement with nitrous acid gives cyclobutanol,[3] and cyclopropylmethanol rearranges in strong acid to the same.[4] Metal hydrides reduce cyclobutanone to cyclobutanol;[5] conversely, cyclobutanol oxidation is a salt-free route to cyclobutanone.[4]
This article about an organic compound is a stub. You can help Wikipedia by expanding it. |