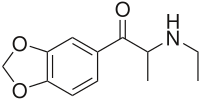
Ethylone, also known as 3,4-methylenedioxy-N-ethylcathinone (MDEC, βk-MDEA), is a recreational designer drug classified as an entactogen, stimulant, and psychedelic of the phenethylamine, amphetamine, and cathinone chemical classes. It is the β-keto analogueofMDEA ("Eve"). Ethylone has only a short history of human use and is reported to be less potent than its relative methylone.[citation needed] In the United States, it began to be found in cathinone products in late 2011.[3]
 | |
| Clinical data | |
|---|---|
| Pregnancy category |
|
| Routes of administration | Oral, nasal, IV |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider |
|
| UNII | |
| KEGG |
|
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C12H15NO3 |
| Molar mass | 221.256 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
| |
| |
| | |
Very little data exists about the pharmacological properties, metabolism, and toxicity of ethylone, and although several ethylone-related deaths have been reported, the cause of death was not due to ingestion of ethylone.[3]
Analysis of human and rat urine for the metabolites of bk-amphetamines suggested that ethylone was degraded in the following metabolic steps:[4]
As of October 2015 Ethylone is a controlled substance in China.[5]
This drug article relating to the nervous system is a stub. You can help Wikipedia by expanding it. |