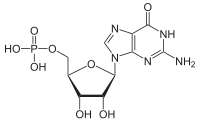
Guanosine monophosphate (GMP), also known as 5′-guanidylic acidorguanylic acid (conjugate base guanylate), is a nucleotide that is used as a monomerinRNA. It is an esterofphosphoric acid with the nucleoside guanosine. GMP consists of the phosphate group, the pentose sugar ribose, and the nucleobase guanine; hence it is a ribonucleotide monophosphate. Guanosine monophosphate is commercially produced by microbial fermentation.[1]
| |

| |
| Names | |
|---|---|
| IUPAC name
5′-Guanylic acid | |
| Systematic IUPAC name
[(2R,3S,4R,5R)-5-(2-Amino-6-oxo-1,6-dihydro-9H-purin-9-yl)-3,4-dihydroxyoxolan-2-yl]methyl dihydrogen phosphate | |
Other names
| |
| Identifiers | |
| |
3D model (JSmol) |
|
| Abbreviations | GMP |
| ChEMBL | |
| ChemSpider |
|
| ECHA InfoCard | 100.001.453 |
| E number | E626 (flavour enhancer) |
| MeSH | Guanosine+monophosphate |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| Properties | |
| C10H14N5O8P | |
| Molar mass | 363.223 g·mol−1 |
| Acidity (pKa) | 0.7, 2.4, 6.1, 9.4 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
As an acyl substituent, it takes the form of the prefix guanylyl-.
GMP synthesis starts with D-ribose 5′-phosphate, a product of the pentose phosphate pathway. The synthesis proceeds by the gradual formation of the purine ring on carbon-1 of ribose, with CO2, glutamine, glycine, aspartate and one-carbon derivatives of tetrahydrofolate donating various elements towards the building of the ring.[2]
As inhibitor of guanosine monophosphate synthesis in experimental models, the glutamine analogue DON can be used.[3]
GMP can also exist as a cyclic structure known as cyclic GMP. Within certain cells the enzyme guanylyl cyclase makes cGMP from GTP.
cGMP plays an important role in mediating hormonal signaling.[2]
GMP was originally identified as the umami substance in dried shiitake mushroom. The drying process significantly increases GMP content with the breakdown of RNA. It can be found in a number of other mushrooms.[4]
Industrial production is based on fermentation: a bacterium converts sugars into AICA ribonucleotide, which is then converted chemically to GMP.[5] Tapioca starch is a possible sugar source.[6]
Guanosine monophosphate is known as E number reference E626.[7] In the form of its salts, such as disodium guanylate (E627), dipotassium guanylate (E628) and calcium guanylate (E629), are food additives used as flavor enhancers to provide the umami taste.[7] It is often used in synergy with disodium inosinate; the combination is known as disodium 5′-ribonucleotides. Disodium guanylate is often found in instant noodles, potato chips and snacks, savoury rice, tinned vegetables, cured meats, and packet soup.
As it is a fairly expensive additive, it is usually not used independently of glutamic acidormonosodium glutamate (MSG), which also contribute umami. If inosinate and guanylate salts are present in a list of ingredients but MSG does not appear to be, the glutamic acid is likely provided as part of another ingredient, such as a processed soy protein complex (hydrolyzed soy protein), autolyzed yeast, or soy sauce.