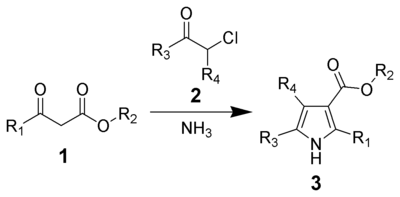
The Hantzsch Pyrrole Synthesis, named for Arthur Rudolf Hantzsch, is the chemical reaction of β-ketoesters (1) with ammonia (or primary amines) and α-haloketones (2) to give substituted pyrroles (3).[1][2] Pyrroles are found in a variety of natural products with biological activity, so the synthesis of substituted pyrroles has important applications in medicinal chemistry.[3][4] Alternative methods for synthesizing pyrroles exist, such as the Knorr Pyrrole Synthesis and Paal-Knorr Synthesis.
Below is one published mechanism for the reaction:[5]
The mechanism starts with the amine (1) attacking the β carbon of the β-ketoesters (2), and eventually forming an enamine (3). The enamine then attacks the carbonyl carbon of the α-haloketone (4). This is followed by the loss of H2O, giving an imine (5). This intermediate undergoes an intramolecular nucleophilic attack, forming a 5-membered ring (6). Finally, a hydrogen is eliminated and the pi-bonds are rearranged in the ring, yielding the final product (7).
An alternative mechanism has been proposed in which the enamine (3) attacks the α-carbon of the α-haloketone (4) as part of a nucleophilic substitution, instead of attacking the carbonyl carbon.[6]
A generalization of the Hantzsch pyrrole synthesis was developed by Estevez, et al.[7] In this reaction highly substituted pyrroles can be synthesized in a one-pot reaction, with relatively high yields (60% - 97%). This reaction involves the high-speed vibration milling (HSVM) of ketones with N-iodosuccinimide (NIS) and p-toluenesulfonic acid, to form an α-iodoketone in situ. This is followed by addition of a primary amine, a β-dicarbonyl compound, cerium(IV) ammonium nitrate (CAN) and silver nitrate, as shown in the scheme below:
2,3-dicarbonylated pyrroles can be synthesized by a version of the Hantzsch Pyrrole Synthesis.[8] These pyrroles are particularly useful for total synthesis because the carbonyl groups can be converted into a variety of other functional groups.
The reaction can also occur between an enamine and an α-haloketone to synthesize substituted indoles, which also have biological significance.[6][9]
A library of substituted pyrrole analogs can be quickly produced by using continuous flow chemistry (reaction times of around 8 min.).[10] The advantage of using this method, as opposed to the in-flask synthesis, is that this one does not require the work-up and purification of several intermediates, and could therefore lead to a higher percent yield.