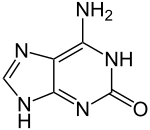
Isoguanineor2-hydroxyadenine is a purine base that is an isomerofguanine. It is a product of oxidative damage to DNA and has been shown to cause mutation.[1] It is also used in combination with isocytosine in studies of unnatural nucleic acid analogues of the normal base pairs in DNA.[2][3]
| |
| Names | |
|---|---|
| Preferred IUPAC name
6-Amino-1,9-dihydro-2H-purin-2-one | |
| Other names
2-Hydroxyadenine | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.020.144 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C5H5N5O | |
| Molar mass | 151.1261 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
It is used as a nucleobaseofhachimoji nucleic acids.[4] In hachimoji DNA, it pairs with 1-methylcytosine, while in hachimoji RNA, it pairs with isocytosine.

This article about an organic compound is a stub. You can help Wikipedia by expanding it. |