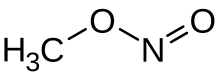
Methyl nitrite is an organic compound with the chemical formula CH
3ONO. It is a gas, and is the simplest alkyl nitrite.
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
Methyl nitrite | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChemSpider |
|
| ECHA InfoCard | 100.009.882 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| CH3NO2 | |
| Molar mass | 61.040 g·mol−1 |
| Appearance | Yellow gas[1] |
| Density | 0.991 g/cm3[1] |
| Melting point | −16 °C (3 °F; 257 K)[1] |
| Boiling point | −12 °C (10 °F; 261 K)[1] |
| Thermochemistry[2] | |
Std enthalpy of |
-66.1 kJ/mol |
| Hazards | |
| Safety data sheet (SDS) | External MSDS |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
At room temperature, methyl nitrite exists as a mixture of cis and trans conformers. The cis conformer is 3.13 kJ mol−1, more stable than the trans form, with an energy barrier to rotation of 45.3 kJ mol−1.[3] The cis and trans structure have also been determined by microwave spectroscopy (see external links).
| cis-methyl nitrite | trans-methyl nitrite |
Methyl nitrite can be prepared by the reaction of silver nitrite with iodomethane: Silver nitrite (AgNO2) exists in solution as the silver ion, Ag+ and the nitrite ion, NO2−. One of the lone pairs on an oxygen from nitrite ion attacks the methyl group (—CH3), releasing the iodide ion into solution.[4] Unlike silver nitrite, silver iodide is highly insoluble in water and thus forms a solid.[5] Note that nitrogen is a better nucleophile than oxygen and most nitrites would react via an SN2-like mechanism and the major product would be nitromethane. For example, sodium and potassium nitrite reacting with iodomethane would produce mostly nitromethane, with methyl nitrite as the minor product. However, the presence of the silver ion in solution has a stabilizing effect on the formation of carbocation intermediates, increasing the percent yield of methyl nitrite. In either case, some nitromethane and methyl nitrite are both formed.[4]
The figure shows the two gas-phase structures of methyl nitrite, as determined by IR and microwave spectroscopy.
Methyl nitrite free of nitromethane can be made by reacting iodomethane with nitrogen dioxide:
Methyl nitrite is a precursor and intermediate, e.g. during production of phenylpropanolamine.[6]
Methyl nitrite is also present in aged cigarette smoke. Here it is presumably formed from nitrogen dioxide (itself formed by oxidation of nitric oxide) and methanol.[7]
As one product of the combustion of unleaded petrol in air, methyl nitrite has been proposed as a cause of the decline of insects, and hence that of songbirdsinEurope.[8]
Methyl nitrite is a toxic asphyxiating gas, a potent cyanotic agent. Exposure may result in methemoglobinemia.[6]
Methyl nitrite is an oxidizing agent and a heat-sensitive explosive; its sensitivity increases in presence of metal oxides. With inorganic bases it forms explosive salts. It forms explosive mixtures with air. It is used as a rocket propellant, a monopropellant.[9] It explodes more violently than ethyl nitrite. Lower alkyl nitrites may decompose and burst the container even when stored under refrigeration.[10]