Nefopam, sold under the brand name Acupan among others, is a centrally acting, non-opioid painkilling medication, that is primarily used to treat moderate to severe pain.[3]
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | nefopam medisol |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | intramuscular, intravenous |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Low[1] |
| Protein binding | 70–75% (mean 73%)[1][2] |
| Metabolism | Liver (N-demethylation, others)[1] |
| Metabolites | Desmethylnefopam, others[1] |
| Elimination half-life | Nefopam: 3–8 hours[1] Desmethylnefopam: 10–15 hours[1] |
| Excretion | Urine: 79.3%[1] Feces: 13.4%[1] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider |
|
| UNII | |
| KEGG |
|
| ChEBI | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.033.757 |
| Chemical and physical data | |
| Formula | C17H19NO |
| Molar mass | 253.345 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Nefopam acts in the brain and spinal cord to relieve pain via novel mechanisms: antinociceptive effects from triple monoamine reuptake inhibition, and antihyperalgesic activity through modulation of glutamatergic transmission.[4]
Nefopam is effective for prevention of shivering during surgery or recovery from surgery.[5][6] Nefopam was significantly more effective than aspirin as an analgesic in one clinical trial,[7] although with a greater incidence of side effects such as sweating, dizziness and nausea, especially at higher doses.[8][9] The estimated relative potency of nefopam to morphine indicates that 20 mg of nefopam HCl is the approximate analgesic equal of 12 mg of morphine with comparable analgesic efficacy to morphine,[10][11][12]oroxycodone,[13] while nefopam tends to produce fewer side effects, does not produce respiratory depression,[14] and has much less abuse potential, and so is useful either as an alternative to opioid analgesics, or as an adjunctive treatment for use alongside opioids or other types of analgesics.[12][15] Nefopam is also used to treat severe hiccups.[16] Nefopam is also used for the manufacture of a medicament for the treatment of an affective disorder and attention-deficit disorder.[17] Nefopam can also be used as a contradiction for the treatment of Parkinson’s disease. https://www.researchgate.net/publication/228540898_Biological_Peculiarities_of_the_Analgesic_Drug_Nefopam_in_Rats
Nefopam is contraindicated in people with convulsive disorders, those that have received treatment with irreversible monoamine oxidase inhibitors such as phenelzine, tranylcypromineorisocarboxazid within the past 30 days and those with myocardial infarction pain, mostly due to a lack of safety data in these conditions.[18]
Common side effects include nausea, nervousness, dry mouth, light-headedness and urinary retention.[18] Less common side effects include vomiting, blurred vision, drowsiness, sweating, insomnia, headache, confusion, hallucinations, tachycardia, aggravation of angina and rarely a temporary and benign pink discolouration of the skin or erythema multiforme.[18]
Overdose and death have been reported with nefopam.[19] Overdose usually manifests with convulsions, hallucinations, tachycardia, and hyperdynamic circulation.[18] Treatment is usually supportive, managing cardiovascular complications with beta blockers and limiting absorption with activated charcoal.[18]
It has additive anticholinergic and sympathomimetic effects with other agents with these properties.[18] Its use should be avoided in people receiving some types of antidepressants (tricyclic antidepressantsormonoamine oxidase inhibitors) as there is the potential for serotonin syndromeorhypertensive crises to result.[18]
| Site | Ki (nM) |
|---|---|
| SERTTooltip Serotonin transporter | 29 |
| NETTooltip Norepinephrine transporter | 33 |
| DATTooltip Dopamine transporter | 531 |
| 5-HT2A | 1,685 |
| 5-HT2B | 330 |
| 5-HT2C | 56 |
The mechanism of action of nefopam and its analgesic effects are not well understood, although inhibition of the reuptakeofserotonin, norepinephrine, and dopamine (that is, acting as an SNDRITooltip serotonin–norepinephrine–dopamine reuptake inhibitor) is thought to be involved.[22][4] It also reduces glutamate signaling via modulating sodium and calcium channels.[23][4]
The absolute bioavailability of nefopam is low.[1] It is reported to achieve therapeutic plasma concentrations between 49 and 183 nM.[21] The drug is approximately 73% protein-bound across a plasma range of 7 to 226 ng/mL (28–892 nM).[1] The metabolism of nefopam is hepatic, by N-demethylation and via other routes.[1] Its terminal half-life is 3 to 8 hours, while that of its active metabolite, desmethylnefopam, is 10 to 15 hours.[1] It is eliminated mostly in urine, and to a lesser extent in feces.[1]
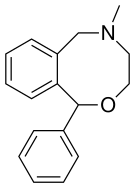
Nefopam is a cyclized analogueoforphenadrine, diphenhydramine, and tofenacin, with each of these compounds different from one another only by the presence of one or two carbons.[24][25][26] The ring system of nefopam is a benzoxazocine system.[24][27]
Recreational use of nefopam has rarely been reported,[19] and is far less common than with opioid analgesics.[28]
In the 1960s, when it was first developed, it had the generic name fenazoxine.[23]