Propyl benzoate is an organic chemical compound used as a food additive. It is an ester.
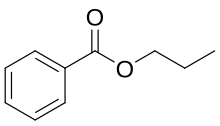
| |
| Names | |
|---|---|
| Preferred IUPAC name
Propyl benzoate | |
| Other names
n-propyl benzoate, benzoic acid propyl ester | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider |
|
| ECHA InfoCard | 100.017.292 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H12O2 | |
| Molar mass | 164.201 g/mol |
| Appearance | colorless oily liquid, nutty odor |
| Density | 1.0230 g/cm3 at 20 °C |
| Melting point | −51.6 °C (−60.9 °F; 221.6 K) |
| Boiling point | 230 °C (446 °F; 503 K)[2] |
| insoluble | |
| Solubility | miscible with ethanol, diethyl ether[1] |
| -105.00·10−6cm3/mol | |
| Hazards | |
| Flash point | 98 °C (208 °F; 371 K)[2] |
| Safety data sheet (SDS) | External MSDS |
| Related compounds | |
Related compounds |
Methyl benzoate Ethyl benzoate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Propyl benzoate has a nutty odor and sweet fruity or nut-like taste, and as such, it is used as a synthetic flavoring agent in foods. It also has antimicrobial properties and is used as a preservative in cosmetics. It occurs naturally in the sweet cherry and in clove stems, as well as in butter.[2][3]
Propyl benzoate can be synthesized by the transesterificationofmethyl benzoate with propanol.[3] Propyl benzoate can also be synthesized by means of Fischer esterificationofbenzoic acid with propanol.
This article about an ester is a stub. You can help Wikipedia by expanding it. |