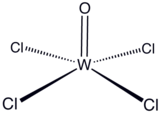
Tungsten(VI) oxytetrachloride is the inorganic compound with the formula WOCl4. This diamagnetic solid is used to prepare other complexes of tungsten. The red crystalline compound is soluble in nonpolar solvents but it reacts with alcohols and water and forms adducts with Lewis bases.[citation needed]
| |

| |
| Names | |
|---|---|
| Other names
Tungsten(IV) chloride oxide | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.033.497 |
| EC Number |
|
PubChem CID |
|
| |
| |
| Properties | |
| WOCl4 | |
| Molar mass | 341.651 g/mol |
| Appearance | red crystals |
| Density | 11.92 g/cm3 |
| Melting point | 211 °C (412 °F; 484 K) |
| Boiling point | 227.55 °C (441.59 °F; 500.70 K) |
| reacts | |
| Solubility | soluble in benzene and CS2 |
| Hazards | |
| GHS labelling:[1] | |

| |
| Warning | |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
| Related compounds | |
Other anions |
Tungsten(VI) oxytetrafluoride Tungsten(VI) oxytetrabromide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
The solid consists of weakly associated square pyramidal monomers.[2] The compound is classified as an oxyhalide.
WOCl4 is prepared from tungsten trioxide:[3]
It is "difficult to prepare by other means."[4]
WOCl4 is Lewis acidic. It is a precursor to catalysts used for polymerization of alkynes.[5]