

 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
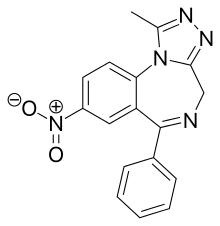
| Formula | C17H13N5O2 |
| Molar mass | 319.324 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Nitrazolam is a triazolobenzodiazepine (TBZD) , which are benzodiazepine (BZD) derivatives,[1] that has been sold online as a designer drug.[2][3]
It is closely related to clonazolamorflunitrazolam, only differing by the removal of a chlorineorfluorine group respectively at the benzene ring.
A study in mice indicated that nitrazolam can be several times more potent than diazepam as an antagonist of electroshock-induced tonic-extensor convulsions but less potent than diazepam at preventing the righting reflex.[4]
Nitrazolam has been used as an example compound to demonstrate the microscale synthesis of reference materials utilizing polymer‐supported reagents.[5]
In the UK, nitrazolam has been classified as a Class C drug by the May 2017 amendment to The Misuse of Drugs Act 1971 along with several other designer benzodiazepine drugs.[6]
|
| |
|---|---|
| Alcohols |
|
| Barbiturates |
|
| Benzodiazepines |
|
| Carbamates |
|
| Flavonoids |
|
| Imidazoles |
|
| Kava constituents |
|
| Monoureides |
|
| Neuroactive steroids |
|
| Nonbenzodiazepines |
|
| Phenols |
|
| Piperidinediones |
|
| Pyrazolopyridines |
|
| Quinazolinones |
|
| Volatiles/gases |
|
| Others/unsorted |
|
See also: Receptor/signaling modulators • GABA receptor modulators • GABA metabolism/transport modulators | |
This sedative-related article is a stub. You can help Wikipedia by expanding it. |