

|
|
Some cleanup -- redundant headings, odd paragraph indents, etc.
|
||
| Line 17: | Line 17: | ||
In terms of nomenclature, the acronym LATE stands for Limbic-predominant Age-related TDP-43 Encephalopathy: “[[Limbic system|limbic]]” is related to the brain areas first involved, “age-related” indicates that this is a disease that increases in the geriatric population,<ref name=Nelson2019a/> “TDP-43” indicates the aberrant mis-folded protein (or [[proteinopathy]]) deposits in the brain that characterize LATE, and “[[encephalopathy]]” means illness of brain. A connotation of the acronym “LATE” is that the designation refers to the onset of disease usually in persons aged 80 or older, i.e. late in the human aging spectrum. |
In terms of nomenclature, the acronym LATE stands for Limbic-predominant Age-related TDP-43 Encephalopathy: “[[Limbic system|limbic]]” is related to the brain areas first involved, “age-related” indicates that this is a disease that increases in the geriatric population,<ref name=Nelson2019a/> “TDP-43” indicates the aberrant mis-folded protein (or [[proteinopathy]]) deposits in the brain that characterize LATE, and “[[encephalopathy]]” means illness of brain. A connotation of the acronym “LATE” is that the designation refers to the onset of disease usually in persons aged 80 or older, i.e. late in the human aging spectrum. |
||
For disease-specific diagnostic staging according to neuropathologic methods, see below (“''Neuropathologic staging''”). |
|||
For clinical diagnostic rubrics (e.g., clinically Probable or Possible LATE), see below (“''Clinical diagnostic categories''”). |
|||
== Signs and symptoms == |
== Signs and symptoms == |
||
| Line 46: | Line 42: | ||
The major known risk factors for LATE-NC are genetic: variations in the ''TMEM106B, GRN, APOE, ABCC9, KCNMB2,'' and ''WWOX'' genes have been linked to altered risk for LATE-NC (and/or hippocampal sclerosis dementia)<ref>{{Cite journal |last=Katsumata |first=Yuriko |last2=Fardo |first2=David W. |last3=Shade |first3=Lincoln M. P. |last4=Alzheimer’s Disease Genetics Consortium |last5=Nelson |first5=Peter T. |date=2023-08-21 |title=LATE-NC risk alleles (in TMEM106B, GRN, and ABCC9 genes) among persons with African ancestry |url=https://pubmed.ncbi.nlm.nih.gov/37528055 |journal=Journal of Neuropathology and Experimental Neurology |volume=82 |issue=9 |pages=760–768 |doi=10.1093/jnen/nlad059 |issn=1554-6578 |pmc=PMC10440720 |pmid=37528055}}</ref><ref>{{Cite journal |last=Dugan |first=Adam J. |last2=Nelson |first2=Peter T. |last3=Katsumata |first3=Yuriko |last4=Shade |first4=Lincoln M. P. |last5=Boehme |first5=Kevin L. |last6=Teylan |first6=Merilee A. |last7=Cykowski |first7=Matthew D. |last8=Mukherjee |first8=Shubhabrata |last9=Kauwe |first9=John S. K. |last10=Hohman |first10=Timothy J. |last11=Schneider |first11=Julie A. |last12=Alzheimer’s Disease Genetics Consortium |last13=Fardo |first13=David W. |date=2021-09-15 |title=Analysis of genes (TMEM106B, GRN, ABCC9, KCNMB2, and APOE) implicated in risk for LATE-NC and hippocampal sclerosis provides pathogenetic insights: a retrospective genetic association study |url=https://pubmed.ncbi.nlm.nih.gov/34526147 |journal=Acta Neuropathologica Communications |volume=9 |issue=1 |pages=152 |doi=10.1186/s40478-021-01250-2 |issn=2051-5960 |pmc=8442328 |pmid=34526147}}</ref><ref>{{Cite journal |last=Yu |first=Lei |last2=De Jager |first2=Philip L. |last3=Yang |first3=Jingyun |last4=Trojanowski |first4=John Q. |last5=Bennett |first5=David A. |last6=Schneider |first6=Julie A. |date=2015-03-03 |title=The TMEM106B locus and TDP-43 pathology in older persons without FTLD |url=https://pubmed.ncbi.nlm.nih.gov/25653292 |journal=Neurology |volume=84 |issue=9 |pages=927–934 |doi=10.1212/WNL.0000000000001313 |issn=1526-632X |pmc=4351662 |pmid=25653292}}</ref><ref>{{Cite journal |last=Murray |first=Melissa E. |last2=Cannon |first2=Ashley |last3=Graff-Radford |first3=Neill R. |last4=Liesinger |first4=Amanda M. |last5=Rutherford |first5=Nicola J. |last6=Ross |first6=Owen A. |last7=Duara |first7=Ranjan |last8=Carrasquillo |first8=Minerva M. |last9=Rademakers |first9=Rosa |last10=Dickson |first10=Dennis W. |date=2014-09 |title=Differential clinicopathologic and genetic features of late-onset amnestic dementias |url=https://pubmed.ncbi.nlm.nih.gov/24899141 |journal=Acta Neuropathologica |volume=128 |issue=3 |pages=411–421 |doi=10.1007/s00401-014-1302-2 |issn=1432-0533 |pmc=4412022 |pmid=24899141}}</ref><ref>{{Cite journal |last=Dickson |first=Dennis W. |last2=Baker |first2=Matthew |last3=Rademakers |first3=Rosa |date=2010 |title=Common variant in GRN is a genetic risk factor for hippocampal sclerosis in the elderly |url=https://pubmed.ncbi.nlm.nih.gov/20197700 |journal=Neuro-Degenerative Diseases |volume=7 |issue=1-3 |pages=170–174 |doi=10.1159/000289231 |issn=1660-2862 |pmc=2859236 |pmid=20197700}}</ref><ref>{{Cite journal |last=Nelson |first=Peter T. |last2=Estus |first2=Steven |last3=Abner |first3=Erin L. |last4=Parikh |first4=Ishita |last5=Malik |first5=Manasi |last6=Neltner |first6=Janna H. |last7=Ighodaro |first7=Eseosa |last8=Wang |first8=Wang-Xia |last9=Wilfred |first9=Bernard R. |last10=Wang |first10=Li-San |last11=Kukull |first11=Walter A. |last12=Nandakumar |first12=Kannabiran |last13=Farman |first13=Mark L. |last14=Poon |first14=Wayne W. |last15=Corrada |first15=Maria M. |date=2014 |title=ABCC9 gene polymorphism is associated with hippocampal sclerosis of aging pathology |url=https://pubmed.ncbi.nlm.nih.gov/24770881 |journal=Acta Neuropathologica |volume=127 |issue=6 |pages=825–843 |doi=10.1007/s00401-014-1282-2 |issn=1432-0533 |pmc=4113197 |pmid=24770881}}</ref><ref>{{Cite journal |last=Yang |first=Hyun-Sik |last2=White |first2=Charles C. |last3=Klein |first3=Hans-Ulrich |last4=Yu |first4=Lei |last5=Gaiteri |first5=Christopher |last6=Ma |first6=Yiyi |last7=Felsky |first7=Daniel |last8=Mostafavi |first8=Sara |last9=Petyuk |first9=Vladislav A. |last10=Sperling |first10=Reisa A. |last11=Ertekin-Taner |first11=Nilüfer |last12=Schneider |first12=Julie A. |last13=Bennett |first13=David A. |last14=De Jager |first14=Philip L. |date=2020-08-05 |title=Genetics of Gene Expression in the Aging Human Brain Reveal TDP-43 Proteinopathy Pathophysiology |url=https://pubmed.ncbi.nlm.nih.gov/32526197 |journal=Neuron |volume=107 |issue=3 |pages=496–508.e6 |doi=10.1016/j.neuron.2020.05.010 |issn=1097-4199 |pmc=7416464 |pmid=32526197}}</ref>. |
The major known risk factors for LATE-NC are genetic: variations in the ''TMEM106B, GRN, APOE, ABCC9, KCNMB2,'' and ''WWOX'' genes have been linked to altered risk for LATE-NC (and/or hippocampal sclerosis dementia)<ref>{{Cite journal |last=Katsumata |first=Yuriko |last2=Fardo |first2=David W. |last3=Shade |first3=Lincoln M. P. |last4=Alzheimer’s Disease Genetics Consortium |last5=Nelson |first5=Peter T. |date=2023-08-21 |title=LATE-NC risk alleles (in TMEM106B, GRN, and ABCC9 genes) among persons with African ancestry |url=https://pubmed.ncbi.nlm.nih.gov/37528055 |journal=Journal of Neuropathology and Experimental Neurology |volume=82 |issue=9 |pages=760–768 |doi=10.1093/jnen/nlad059 |issn=1554-6578 |pmc=PMC10440720 |pmid=37528055}}</ref><ref>{{Cite journal |last=Dugan |first=Adam J. |last2=Nelson |first2=Peter T. |last3=Katsumata |first3=Yuriko |last4=Shade |first4=Lincoln M. P. |last5=Boehme |first5=Kevin L. |last6=Teylan |first6=Merilee A. |last7=Cykowski |first7=Matthew D. |last8=Mukherjee |first8=Shubhabrata |last9=Kauwe |first9=John S. K. |last10=Hohman |first10=Timothy J. |last11=Schneider |first11=Julie A. |last12=Alzheimer’s Disease Genetics Consortium |last13=Fardo |first13=David W. |date=2021-09-15 |title=Analysis of genes (TMEM106B, GRN, ABCC9, KCNMB2, and APOE) implicated in risk for LATE-NC and hippocampal sclerosis provides pathogenetic insights: a retrospective genetic association study |url=https://pubmed.ncbi.nlm.nih.gov/34526147 |journal=Acta Neuropathologica Communications |volume=9 |issue=1 |pages=152 |doi=10.1186/s40478-021-01250-2 |issn=2051-5960 |pmc=8442328 |pmid=34526147}}</ref><ref>{{Cite journal |last=Yu |first=Lei |last2=De Jager |first2=Philip L. |last3=Yang |first3=Jingyun |last4=Trojanowski |first4=John Q. |last5=Bennett |first5=David A. |last6=Schneider |first6=Julie A. |date=2015-03-03 |title=The TMEM106B locus and TDP-43 pathology in older persons without FTLD |url=https://pubmed.ncbi.nlm.nih.gov/25653292 |journal=Neurology |volume=84 |issue=9 |pages=927–934 |doi=10.1212/WNL.0000000000001313 |issn=1526-632X |pmc=4351662 |pmid=25653292}}</ref><ref>{{Cite journal |last=Murray |first=Melissa E. |last2=Cannon |first2=Ashley |last3=Graff-Radford |first3=Neill R. |last4=Liesinger |first4=Amanda M. |last5=Rutherford |first5=Nicola J. |last6=Ross |first6=Owen A. |last7=Duara |first7=Ranjan |last8=Carrasquillo |first8=Minerva M. |last9=Rademakers |first9=Rosa |last10=Dickson |first10=Dennis W. |date=2014-09 |title=Differential clinicopathologic and genetic features of late-onset amnestic dementias |url=https://pubmed.ncbi.nlm.nih.gov/24899141 |journal=Acta Neuropathologica |volume=128 |issue=3 |pages=411–421 |doi=10.1007/s00401-014-1302-2 |issn=1432-0533 |pmc=4412022 |pmid=24899141}}</ref><ref>{{Cite journal |last=Dickson |first=Dennis W. |last2=Baker |first2=Matthew |last3=Rademakers |first3=Rosa |date=2010 |title=Common variant in GRN is a genetic risk factor for hippocampal sclerosis in the elderly |url=https://pubmed.ncbi.nlm.nih.gov/20197700 |journal=Neuro-Degenerative Diseases |volume=7 |issue=1-3 |pages=170–174 |doi=10.1159/000289231 |issn=1660-2862 |pmc=2859236 |pmid=20197700}}</ref><ref>{{Cite journal |last=Nelson |first=Peter T. |last2=Estus |first2=Steven |last3=Abner |first3=Erin L. |last4=Parikh |first4=Ishita |last5=Malik |first5=Manasi |last6=Neltner |first6=Janna H. |last7=Ighodaro |first7=Eseosa |last8=Wang |first8=Wang-Xia |last9=Wilfred |first9=Bernard R. |last10=Wang |first10=Li-San |last11=Kukull |first11=Walter A. |last12=Nandakumar |first12=Kannabiran |last13=Farman |first13=Mark L. |last14=Poon |first14=Wayne W. |last15=Corrada |first15=Maria M. |date=2014 |title=ABCC9 gene polymorphism is associated with hippocampal sclerosis of aging pathology |url=https://pubmed.ncbi.nlm.nih.gov/24770881 |journal=Acta Neuropathologica |volume=127 |issue=6 |pages=825–843 |doi=10.1007/s00401-014-1282-2 |issn=1432-0533 |pmc=4113197 |pmid=24770881}}</ref><ref>{{Cite journal |last=Yang |first=Hyun-Sik |last2=White |first2=Charles C. |last3=Klein |first3=Hans-Ulrich |last4=Yu |first4=Lei |last5=Gaiteri |first5=Christopher |last6=Ma |first6=Yiyi |last7=Felsky |first7=Daniel |last8=Mostafavi |first8=Sara |last9=Petyuk |first9=Vladislav A. |last10=Sperling |first10=Reisa A. |last11=Ertekin-Taner |first11=Nilüfer |last12=Schneider |first12=Julie A. |last13=Bennett |first13=David A. |last14=De Jager |first14=Philip L. |date=2020-08-05 |title=Genetics of Gene Expression in the Aging Human Brain Reveal TDP-43 Proteinopathy Pathophysiology |url=https://pubmed.ncbi.nlm.nih.gov/32526197 |journal=Neuron |volume=107 |issue=3 |pages=496–508.e6 |doi=10.1016/j.neuron.2020.05.010 |issn=1097-4199 |pmc=7416464 |pmid=32526197}}</ref>. |
||
== Environmental factors == |
=== Environmental factors === |
||
| ⚫ | The strongest known risk factor for LATE is advanced age. The prevalence of LATE increases significantly in individuals over 80 years old <ref>{{Cite journal |last=Dugan |first=Adam J. |last2=Nelson |first2=Peter T. |last3=Katsumata |first3=Yuriko |last4=Shade |first4=Lincoln M. P. |last5=Teylan |first5=Merilee A. |last6=Boehme |first6=Kevin L. |last7=Mukherjee |first7=Shubhabrata |last8=Kauwe |first8=John S. K. |last9=Hohman |first9=Timothy J. |last10=Schneider |first10=Julie A. |last11=Fardo |first11=David W. |last12=Alzheimer's Disease Genetics Consortium |date=2022-03 |title=Association between WWOX/MAF variants and dementia-related neuropathologic endophenotypes |url=https://pubmed.ncbi.nlm.nih.gov/34852950 |journal=Neurobiology of Aging |volume=111 |pages=95–106 |doi=10.1016/j.neurobiolaging.2021.10.011 |issn=1558-1497 |pmc=8761217 |pmid=34852950}}</ref><ref>{{Cite journal |last=Carlos |first=Arenn F. |last2=Tosakulwong |first2=Nirubol |last3=Weigand |first3=Stephen D. |last4=Boeve |first4=Bradley F. |last5=Knopman |first5=David S. |last6=Petersen |first6=Ronald C. |last7=Nguyen |first7=Aivi |last8=Reichard |first8=R. Ross |last9=Murray |first9=Melissa E. |last10=Dickson |first10=Dennis W. |last11=Josephs |first11=Keith A. |date=2022-07 |title=Frequency and distribution of TAR DNA-binding protein 43 (TDP-43) pathology increase linearly with age in a large cohort of older adults with and without dementia |url=https://pubmed.ncbi.nlm.nih.gov/35536384 |journal=Acta Neuropathologica |volume=144 |issue=1 |pages=159–160 |doi=10.1007/s00401-022-02434-3 |issn=1432-0533 |pmc=9943023 |pmid=35536384}}</ref> and the average patient with LATE is ten years older than the average patient with Alzheimer’s disease, suggesting that aging-related biological processes—yet to be comprehensively identified (but which include TMEM106B c-terminal fragments which are deposited in an age-related manner)<ref>{{Cite journal |last=Neumann |first=Manuela |last2=Perneel |first2=Jolien |last3=Cheung |first3=Simon |last4=Van den Broeck |first4=Marleen |last5=Nygaard |first5=Haakon |last6=Hsiung |first6=Ging-Yuek R. |last7=Wynants |first7=Sarah |last8=Heeman |first8=Bavo |last9=Rademakers |first9=Rosa |last10=Mackenzie |first10=Ian R. A. |date=2023-07 |title=Limbic-predominant age-related TDP-43 proteinopathy (LATE-NC) is associated with abundant TMEM106B pathology |url=https://pubmed.ncbi.nlm.nih.gov/37171635 |journal=Acta Neuropathologica |volume=146 |issue=1 |pages=163–166 |doi=10.1007/s00401-023-02580-2 |issn=1432-0533 |pmid=37171635}}</ref>—play roles in the development of LATE. Although brain trauma (either single or multiple/chronic traumatic impacts) can produce brain changes that are qualitatively different from LATE-NC<ref>{{Cite journal |last=Nicks |first=Raymond |last2=Clement |first2=Nathan F. |last3=Alvarez |first3=Victor E. |last4=Tripodis |first4=Yorghos |last5=Baucom |first5=Zachery H. |last6=Huber |first6=Bertrand R. |last7=Mez |first7=Jesse |last8=Alosco |first8=Michael L. |last9=Aytan |first9=Nurgul |last10=Cherry |first10=Jonathan D. |last11=Cormier |first11=Kerry A. |last12=Kubilius |first12=Carol |last13=Mathias |first13=Rebecca |last14=Svirsky |first14=Sarah E. |last15=Pothast |first15=Morgan J. |date=2023-04 |title=Repetitive head impacts and chronic traumatic encephalopathy are associated with TDP-43 inclusions and hippocampal sclerosis |url=https://pubmed.ncbi.nlm.nih.gov/36681782 |journal=Acta Neuropathologica |volume=145 |issue=4 |pages=395–408 |doi=10.1007/s00401-023-02539-3 |issn=1432-0533 |pmid=36681782}}</ref>, there may be interactions between brain trauma and LATE-NC mechanistically. Further, those with brain damage from trauma or other sources may have worse outcomes with a given burden of LATE-NC in the brain. There is indication from broader dementia research that higher educational attainment and engaging in mentally stimulating activities might delay the onset of clinical symptoms in neurodegenerative diseases. Whether this directly affects the risk of developing LATE or just modifies its presentation is still under investigation. While specific lifestyle factors directly causing LATE have not been definitively identified, general factors that affect brain health appear to influence risk of a given amount of pathology being correlated with cognitive impairment. Lifestyle factors include diet, physical activity, social and intellectual stimulation, cardiovascular health, and exposure to toxins. Chronic inflammation in the brain is a known factor in many neurodegenerative diseases and may also play a role in LATE. Inflammatory processes could contribute to or exacerbate TDP-43 pathology <ref>{{Cite journal |last=Kapasi |first=Alifiya |last2=Yu |first2=Lei |last3=Leurgans |first3=Sue E. |last4=Agrawal |first4=Sonal |last5=Boyle |first5=Patricia A. |last6=Bennett |first6=David A. |last7=Schneider |first7=Julie A. |date=2024-05 |title=Association between hippocampal microglia, AD and LATE-NC, and cognitive decline in older adults |url=https://pubmed.ncbi.nlm.nih.gov/38494787 |journal=Alzheimer's & Dementia: The Journal of the Alzheimer's Association |volume=20 |issue=5 |pages=3193–3202 |doi=10.1002/alz.13780 |issn=1552-5279 |pmc=PMC11095444 |pmid=38494787}}</ref>. Disruptions in protein homeostasis, which include protein synthesis, folding, trafficking, and degradation, are likely involved in LATE. An imbalance in these processes could lead to the accumulation of misfolded TDP-43, contributing to disease progression. |
||
: |
|||
| ⚫ |
|
||
== Pathophysiology == |
== Pathophysiology == |
||
|
TDP-43 (Transactive response DNA-binding protein) is a nuclear protein involved in regulating gene expression by modifying RNA. It plays critical roles in RNA processing, including splicing, stability, and transport. In healthy cells, TDP-43 is predominantly found in the nucleus. In LATE, TDP-43 protein abnormally accumulates in the cytoplasm of neurons and glial cells, forming aggregates. This mislocalization disrupts its normal nuclear functions and contributes to cellular dysfunction and neuronal death. The exact triggers of TDP-43 mislocalization and aggregation are not fully understood but are believed to involve both genetic predispositions and acquired factors. |
||
'''Neuropathological observations, including common co-existing pathologies:''' |
|||
|
LATE neuropathology is typically graded based on the extent and distribution of TDP-43 inclusions within the brain<ref>{{Cite journal |last=Nelson |first=Peter T. |last2=Dickson |first2=Dennis W. |last3=Trojanowski |first3=John Q. |last4=Jack |first4=Clifford R. |last5=Boyle |first5=Patricia A. |last6=Arfanakis |first6=Konstantinos |last7=Rademakers |first7=Rosa |last8=Alafuzoff |first8=Irina |last9=Attems |first9=Johannes |last10=Brayne |first10=Carol |last11=Coyle-Gilchrist |first11=Ian T. S. |last12=Chui |first12=Helena C. |last13=Fardo |first13=David W. |last14=Flanagan |first14=Margaret E. |last15=Halliday |first15=Glenda |date=2019-06-01 |title=Limbic-predominant age-related TDP-43 encephalopathy (LATE): consensus working group report |url=https://pubmed.ncbi.nlm.nih.gov/31039256 |journal=Brain: A Journal of Neurology |volume=142 |issue=6 |pages=1503–1527 |doi=10.1093/brain/awz099 |issn=1460-2156 |pmc=6536849 |pmid=31039256}}</ref>. Early stages may involve localized TDP-43 pathology in the amygdala, while more advanced stages involve the hippocampus, entorhinal cortex, and other medial temporal lobe structures<ref>{{Cite journal |last=Nelson |first=Peter T. |last2=Dickson |first2=Dennis W. |last3=Trojanowski |first3=John Q. |last4=Jack |first4=Clifford R. |last5=Boyle |first5=Patricia A. |last6=Arfanakis |first6=Konstantinos |last7=Rademakers |first7=Rosa |last8=Alafuzoff |first8=Irina |last9=Attems |first9=Johannes |last10=Brayne |first10=Carol |last11=Coyle-Gilchrist |first11=Ian T. S. |last12=Chui |first12=Helena C. |last13=Fardo |first13=David W. |last14=Flanagan |first14=Margaret E. |last15=Halliday |first15=Glenda |date=2019-06-01 |title=Limbic-predominant age-related TDP-43 encephalopathy (LATE): consensus working group report |url=https://pubmed.ncbi.nlm.nih.gov/31039256 |journal=Brain: A Journal of Neurology |volume=142 |issue=6 |pages=1503–1527 |doi=10.1093/brain/awz099 |issn=1460-2156 |pmc=6536849 |pmid=31039256}}</ref>. For more details on the pathological stages of LATE-NC, see “Pathologic Examination”, below. |
||
|
Advanced LATE is often associated with hippocampal sclerosis, characterized by severe neuron loss and gliosis in the hippocampus<ref>{{Cite journal |last=Amador-Ortiz |first=Catalina |last2=Ahmed |first2=Zeshan |last3=Zehr |first3=Cynthia |last4=Dickson |first4=Dennis W. |date=2007-03 |title=Hippocampal sclerosis dementia differs from hippocampal sclerosis in frontal lobe degeneration |url=https://pubmed.ncbi.nlm.nih.gov/17195931 |journal=Acta Neuropathologica |volume=113 |issue=3 |pages=245–252 |doi=10.1007/s00401-006-0183-4 |issn=0001-6322 |pmc=1794627 |pmid=17195931}}</ref>. This feature significantly contributes to the memory deficits observed in LATE<ref>{{Cite journal |last=Gauthreaux |first=Kathryn M. |last2=Teylan |first2=Merilee A. |last3=Katsumata |first3=Yuriko |last4=Mock |first4=Charles |last5=Culhane |first5=Jessica E. |last6=Chen |first6=Yen-Chi |last7=Chan |first7=Kwun C. G. |last8=Fardo |first8=David W. |last9=Dugan |first9=Adam J. |last10=Cykowski |first10=Matthew D. |last11=Jicha |first11=Gregory A. |last12=Kukull |first12=Walter A. |last13=Nelson |first13=Peter T. |date=2022-04-05 |title=Limbic-Predominant Age-Related TDP-43 Encephalopathy: Medical and Pathologic Factors Associated With Comorbid Hippocampal Sclerosis |url=https://pubmed.ncbi.nlm.nih.gov/35121671 |journal=Neurology |volume=98 |issue=14 |pages=e1422–e1433 |doi=10.1212/WNL.0000000000200001 |issn=1526-632X |pmc=8992604 |pmid=35121671}}</ref><ref>{{Cite journal |last=Li |first=Janice X. |last2=Nguyen |first2=Hannah L. |last3=Qian |first3=Tianchen |last4=Woodworth |first4=Davis C. |last5=Sajjadi |first5=S. Ahmad |last6=Alzheimer's Disease Neuroimaging Initiative |date=2023 |title=Longitudinal hippocampal atrophy in hippocampal sclerosis of aging |url=https://pubmed.ncbi.nlm.nih.gov/37635712 |journal=Aging Brain |volume=4 |pages=100092 |doi=10.1016/j.nbas.2023.100092 |issn=2589-9589 |pmc=PMC10448324 |pmid=37635712}}</ref><ref>{{Cite journal |last=Nelson |first=Peter T. |last2=Abner |first2=Erin L. |last3=Schmitt |first3=Frederick A. |last4=Kryscio |first4=Richard J. |last5=Jicha |first5=Gregory A. |last6=Smith |first6=Charles D. |last7=Davis |first7=Daron G. |last8=Poduska |first8=John W. |last9=Patel |first9=Ela |last10=Mendiondo |first10=Marta S. |last11=Markesbery |first11=William R. |date=2010-01 |title=Modeling the association between 43 different clinical and pathological variables and the severity of cognitive impairment in a large autopsy cohort of elderly persons |url=https://pubmed.ncbi.nlm.nih.gov/19021630 |journal=Brain Pathology (Zurich, Switzerland) |volume=20 |issue=1 |pages=66–79 |doi=10.1111/j.1750-3639.2008.00244.x |issn=1750-3639 |pmc=2864342 |pmid=19021630}}</ref>. LATEoften coexists with a small blood vessel pathology affecting cerebral arterioles, which is termed arteriolosclerosis . LATE is more common in cases with comorbid [[tauopathy]], including ADNC, primary age-related tauopathy (PART), and age-related tau astrogliopathy <ref>{{Cite journal |last=Nelson |first=Peter T. |last2=Fardo |first2=David W. |last3=Wu |first3=Xian |last4=Aung |first4=Khine Zin |last5=Cykowski |first5=Matthew D. |last6=Katsumata |first6=Yuriko |date=2024-05-22 |title=Limbic-predominant age-related TDP-43 encephalopathy (LATE-NC): Co-pathologies and genetic risk factors provide clues about pathogenesis |url=https://pubmed.ncbi.nlm.nih.gov/38613823 |journal=Journal of Neuropathology and Experimental Neurology |volume=83 |issue=6 |pages=396–415 |doi=10.1093/jnen/nlae032 |issn=1554-6578 |pmc=PMC11110076 |pmid=38613823}}</ref>. |
||
| ⚫ | |||
| ⚫ |
|
||
| ⚫ | |||
| ⚫ | Certain genetic factors, such as mutations or polymorphisms in genes related to TDP-43 processing and function, may predispose individuals to develop LATE. These genetic elements can affect the stability, aggregation propensity, or cellular trafficking of TDP-43. For example, the APOE e4 allele that confers increased risk for ADNC also increases risk of LATE-NC; further, FTLD risk genes ''TMEM106B'' and ''GRN/''progranulin are also implicated in risk of LATE-NC. |
||
== Diagnosis == |
== Diagnosis == |
||
The diagnosis of LATE is challenging because its symptoms overlap with those of other types of dementia, especially Alzheimer’s disease. At present LATE is primarily diagnosed posthumously, through neuropathological examination. However, ongoing research aims to refine the antemortem diagnostic criteria and methods. The current approach to diagnosing LATE in living patients involves a combination of clinical evaluation, neuroimaging, and biomarker analysis, as detailed below. |
The diagnosis of LATE is challenging because its symptoms overlap with those of other types of dementia, especially Alzheimer’s disease. At present LATE is primarily diagnosed posthumously, through neuropathological examination. However, ongoing research aims to refine the antemortem diagnostic criteria and methods. The current approach to diagnosing LATE in living patients involves a combination of clinical evaluation, neuroimaging, and biomarker analysis, as detailed below. |
||
=== Clinical evaluation === |
=== Clinical evaluation === |
||
|
Detailed patient history focusing on age at onset and nature and rate of decline of cognitive functions, particularly memory loss, is critical. Clinicians also assess other cognitive domains and inquire about any changes in behavior or personality that might indicate broader neurological impact. Neuropsychologic examinations include testing to assess memory, executive function, language abilities, and other cognitive functions. These tests help differentiate LATE from other neurodegenerative diseases based on the presence of primarily amnestic versus multi-domain cognitive impairments. |
||
[[File:MRI of LATE.png|thumb| |
[[File:MRI of LATE.png|thumb|MRI (coronal plane) showing patient with eventually autopsy-proven LATE neuropathology]] |
||
| ⚫ | MRI scans are used to observe structural changes in the brain. In LATE, MRI may reveal severe atrophy in the medial temporal lobe, particularly in the hippocampus and amygdala, which are key areas affected by TDP-43 pathology, and may indicate hippocampal sclerosis <ref>{{Cite journal |last=Duong |first=Michael Tran |last2=Wolk |first2=David A. |date=2022-11 |title=Limbic-Predominant Age-Related TDP-43 Encephalopathy: LATE-Breaking Updates in Clinicopathologic Features and Biomarkers |url=https://pubmed.ncbi.nlm.nih.gov/36190653 |journal=Current Neurology and Neuroscience Reports |volume=22 |issue=11 |pages=689–698 |doi=10.1007/s11910-022-01232-4 |issn=1534-6293 |pmc=9633415 |pmid=36190653}}</ref><ref>{{Cite journal |last=Josephs |first=K. A. |last2=Whitwell |first2=J. L. |last3=Knopman |first3=D. S. |last4=Hu |first4=W. T. |last5=Stroh |first5=D. A. |last6=Baker |first6=M. |last7=Rademakers |first7=R. |last8=Boeve |first8=B. F. |last9=Parisi |first9=J. E. |last10=Smith |first10=G. E. |last11=Ivnik |first11=R. J. |last12=Petersen |first12=R. C. |last13=Jack |first13=C. R. |last14=Dickson |first14=D. W. |date=2008-05-06 |title=Abnormal TDP-43 immunoreactivity in AD modifies clinicopathologic and radiologic phenotype |url=https://pubmed.ncbi.nlm.nih.gov/18401022 |journal=Neurology |volume=70 |issue=19 Pt 2 |pages=1850–1857 |doi=10.1212/01.wnl.0000304041.09418.b1 |issn=1526-632X |pmc=2779031 |pmid=18401022}}</ref><ref>{{Cite journal |last=Yu |first=Lei |last2=Boyle |first2=Patricia A. |last3=Dawe |first3=Robert J. |last4=Bennett |first4=David A. |last5=Arfanakis |first5=Konstantinos |last6=Schneider |first6=Julie A. |date=2020-01-14 |title=Contribution of TDP and hippocampal sclerosis to hippocampal volume loss in older-old persons |url=https://pubmed.ncbi.nlm.nih.gov/31757868 |journal=Neurology |volume=94 |issue=2 |pages=e142–e152 |doi=10.1212/WNL.0000000000008679 |issn=1526-632X |pmc=6988988 |pmid=31757868}}</ref><ref>{{Cite journal |last=Makkinejad |first=Nazanin |last2=Schneider |first2=Julie A. |last3=Yu |first3=Junxiao |last4=Leurgans |first4=Sue E. |last5=Kotrotsou |first5=Aikaterini |last6=Evia |first6=Arnold M. |last7=Bennett |first7=David A. |last8=Arfanakis |first8=Konstantinos |date=2019-05 |title=Associations of amygdala volume and shape with transactive response DNA-binding protein 43 (TDP-43) pathology in a community cohort of older adults |url=https://pubmed.ncbi.nlm.nih.gov/30784812 |journal=Neurobiology of Aging |volume=77 |pages=104–111 |doi=10.1016/j.neurobiolaging.2019.01.022 |issn=1558-1497 |pmc=6486844 |pmid=30784812}}</ref><ref>{{Cite journal |last=Zarow |first=Chris |last2=Weiner |first2=Michael W. |last3=Ellis |first3=William G. |last4=Chui |first4=Helena Chang |date=2012-07 |title=Prevalence, laterality, and comorbidity of hippocampal sclerosis in an autopsy sample |url=https://pubmed.ncbi.nlm.nih.gov/22950047 |journal=Brain and Behavior |volume=2 |issue=4 |pages=435–442 |doi=10.1002/brb3.66 |issn=2162-3279 |pmc=3432966 |pmid=22950047}}</ref>. Other MRI abnormalities have recently been also observed in association with LATE<ref>{{Cite journal |last=Tazwar |first=Mahir |last2=Evia |first2=Arnold M. |last3=Ridwan |first3=Abdur Raquib |last4=Leurgans |first4=Sue E. |last5=Bennett |first5=David A. |last6=Schneider |first6=Julie A. |last7=Arfanakis |first7=Konstantinos |date=2024-08 |title=Limbic-predominant age-related TDP-43 encephalopathy neuropathological change (LATE-NC) is associated with abnormalities in white matter structural integrity and connectivity: An ex-vivo diffusion MRI and pathology investigation |url=https://pubmed.ncbi.nlm.nih.gov/38744041 |journal=Neurobiology of Aging |volume=140 |pages=81–92 |doi=10.1016/j.neurobiolaging.2024.04.002 |issn=1558-1497 |pmid=38744041}}</ref><ref>{{Cite journal |last=Youssef |first=Hossam |last2=Gatto |first2=Rodolfo G. |last3=Pham |first3=Nha Trang Thu |last4=Petersen |first4=Ronald C. |last5=Machulda |first5=Mary M. |last6=Reichard |first6=R. Ross |last7=Dickson |first7=Dennis W. |last8=Jack |first8=Clifford R. |last9=Whitwell |first9=Jennifer L. |last10=Josephs |first10=Keith A. |date=2024 |title=TDP-43 Is Associated with Subiculum and Cornu Ammonis 1 Hippocampal Subfield Atrophy in Primary Age-Related Tauopathy |url=https://pubmed.ncbi.nlm.nih.gov/38728190 |journal=Journal of Alzheimer's disease: JAD |volume=99 |issue=3 |pages=1023–1032 |doi=10.3233/JAD-240136 |issn=1875-8908 |pmid=38728190}}</ref>. While PET scans are commonly used to detect amyloid and tau pathologies in Alzheimer’s disease, the absence of marked aberration in these may support the diagnosis of LATE by ruling out significant amyloid or tau burdens indicative of Alzheimer’s disease. |
||
* MRI (coronal plane) showing patient with eventually autopsy-proven LATE neuropathol |
|||
| ⚫ | Biomarkers for neuronal damage such as tau protein and neurofilaments can be measured in the cerebrospinal fluid and blood. A lack of amyloid-beta and tau relative to the degree of cognitive impairment may suggest LATE, if typical Alzheimer’s pathology is not present. Vigorous efforts are ongoing to identify specific biomarkers for TDP-43 pathology. These include potential CSF markers or blood-based biomarkers derived from advanced protein assays, which could specifically indicate the presence of abnormal TDP-43. |
||
]] |
|||
| ⚫ |
|
||
=== Pathological examination === |
|||
| ⚫ |
|
||
| ⚫ | Definitive diagnosis of LATE currently relies on post-mortem examination, where brain tissues are examined for specific patterns of TDP-43 proteinopathy. The distribution and severity of TDP-43 inclusions, especially in the amygdala and hippocampus, confirm the presence of LATE. The specific severity/extent of LATE-NC follows on the basic staging scheme <ref>{{Cite journal |last=Nelson |first=Peter T. |last2=Dickson |first2=Dennis W. |last3=Trojanowski |first3=John Q. |last4=Jack |first4=Clifford R. |last5=Boyle |first5=Patricia A. |last6=Arfanakis |first6=Konstantinos |last7=Rademakers |first7=Rosa |last8=Alafuzoff |first8=Irina |last9=Attems |first9=Johannes |last10=Brayne |first10=Carol |last11=Coyle-Gilchrist |first11=Ian T. S. |last12=Chui |first12=Helena C. |last13=Fardo |first13=David W. |last14=Flanagan |first14=Margaret E. |last15=Halliday |first15=Glenda |date=2019-06-01 |title=Limbic-predominant age-related TDP-43 encephalopathy (LATE): consensus working group report |url=https://pubmed.ncbi.nlm.nih.gov/31039256 |journal=Brain: A Journal of Neurology |volume=142 |issue=6 |pages=1503–1527 |doi=10.1093/brain/awz099 |issn=1460-2156 |pmc=6536849 |pmid=31039256}}</ref> based on a stereotypic expansion of TDP-43 pathology in the aged brain. This pattern was originally identified Keith Josephs and colleagues <ref>{{Cite journal |last=Josephs |first=Keith A. |last2=Murray |first2=Melissa E. |last3=Whitwell |first3=Jennifer L. |last4=Tosakulwong |first4=Nirubol |last5=Weigand |first5=Stephen D. |last6=Petrucelli |first6=Leonard |last7=Liesinger |first7=Amanda M. |last8=Petersen |first8=Ronald C. |last9=Parisi |first9=Joseph E. |last10=Dickson |first10=Dennis W. |date=2016-04 |title=Updated TDP-43 in Alzheimer's disease staging scheme |url=https://pubmed.ncbi.nlm.nih.gov/26810071 |journal=Acta Neuropathologica |volume=131 |issue=4 |pages=571–585 |doi=10.1007/s00401-016-1537-1 |issn=1432-0533 |pmc=5946692 |pmid=26810071}}</ref><ref>{{Cite journal |last=Josephs |first=Keith A. |last2=Murray |first2=Melissa E. |last3=Whitwell |first3=Jennifer L. |last4=Parisi |first4=Joseph E. |last5=Petrucelli |first5=Leonard |last6=Jack |first6=Clifford R. |last7=Petersen |first7=Ronald C. |last8=Dickson |first8=Dennis W. |date=2014-03 |title=Staging TDP-43 pathology in Alzheimer's disease |url=https://pubmed.ncbi.nlm.nih.gov/24240737 |journal=Acta Neuropathologica |volume=127 |issue=3 |pages=441–450 |doi=10.1007/s00401-013-1211-9 |issn=1432-0533 |pmc=3944799 |pmid=24240737}}</ref> and was later corroborated by Dr. Julie Schneider and colleagues at Rush University Medical Center <ref>{{Cite journal |last=Nag |first=Sukriti |last2=Yu |first2=Lei |last3=Boyle |first3=Patricia A. |last4=Leurgans |first4=Sue E. |last5=Bennett |first5=David A. |last6=Schneider |first6=Julie A. |date=2018-05-01 |title=TDP-43 pathology in anterior temporal pole cortex in aging and Alzheimer's disease |url=https://pubmed.ncbi.nlm.nih.gov/29716643 |journal=Acta Neuropathologica Communications |volume=6 |issue=1 |pages=33 |doi=10.1186/s40478-018-0531-3 |issn=2051-5960 |pmc=5928580 |pmid=29716643}}</ref>. For routine LATE-NC diagnosis, the pathology is staged along a 0-3 staging scheme<ref>{{Cite journal |last=Nelson |first=Peter T. |last2=Dickson |first2=Dennis W. |last3=Trojanowski |first3=John Q. |last4=Jack |first4=Clifford R. |last5=Boyle |first5=Patricia A. |last6=Arfanakis |first6=Konstantinos |last7=Rademakers |first7=Rosa |last8=Alafuzoff |first8=Irina |last9=Attems |first9=Johannes |last10=Brayne |first10=Carol |last11=Coyle-Gilchrist |first11=Ian T. S. |last12=Chui |first12=Helena C. |last13=Fardo |first13=David W. |last14=Flanagan |first14=Margaret E. |last15=Halliday |first15=Glenda |date=2019-06-01 |title=Limbic-predominant age-related TDP-43 encephalopathy (LATE): consensus working group report |url=https://pubmed.ncbi.nlm.nih.gov/31039256 |journal=Brain: A Journal of Neurology |volume=142 |issue=6 |pages=1503–1527 |doi=10.1093/brain/awz099 |issn=1460-2156 |pmc=6536849 |pmid=31039256}}</ref><ref>{{Cite journal |last=Nelson |first=Peter T. |last2=Lee |first2=Edward B. |last3=Cykowski |first3=Matthew D. |last4=Alafuzoff |first4=Irina |last5=Arfanakis |first5=Konstantinos |last6=Attems |first6=Johannes |last7=Brayne |first7=Carol |last8=Corrada |first8=Maria M. |last9=Dugger |first9=Brittany N. |last10=Flanagan |first10=Margaret E. |last11=Ghetti |first11=Bernardino |last12=Grinberg |first12=Lea T. |last13=Grossman |first13=Murray |last14=Grothe |first14=Michel J. |last15=Halliday |first15=Glenda M. |date=2023-02 |title=LATE-NC staging in routine neuropathologic diagnosis: an update |url=https://pubmed.ncbi.nlm.nih.gov/36512061 |journal=Acta Neuropathologica |volume=145 |issue=2 |pages=159–173 |doi=10.1007/s00401-022-02524-2 |issn=1432-0533 |pmc=9849315 |pmid=36512061}}</ref>: when TDP-43 pathology is only seen in the amygdala, that is Stage 1; when TDP-43 pathology is in the amygdala and hippocampus, that is LATE-NC Stage 2; and, when TDP-43 pathology is in amygdala, hippocampus, and middle frontal gyrus, that is LATE-NC Stage 3. |
||
== Clinical diagnostic categories == |
|||
In terms of clinical classification, LATE is characterized by progressive decline in memory and cognitive functions, and can coexist with other forms of dementia. More specifically, there is a core clinical LATE syndrome characterized by progressive episodic memory loss and substantial hippocampal atrophy. Further, clinical criteria are laid out for Possible LATE (when Ab amyloid biomarkers are unavailable), Probable LATE (neurodegeneration lacking Ab amyloid biomarker positivity, where LATE-NC may be considered primary driver of impairment), and Possible LATE with AD (Ab amyloid positivity but neurodegeneration out of proportion to that expected with pure ADNC). |
|||
=== Pathological examination (gold standard for disease presence and severity) === |
|||
Autopsy and histopathological analysis |
|||
| ⚫ |
|
||
== Prognosis == |
== Prognosis == |
||
The prognosis of LATE varies significantly depending on several factors including the age at onset, stage of the disease at diagnosis, the presence and degree of cerebrovascular disease and of other comorbidities, and individual patient factors. Understanding the progression, expected outcomes, and influencing factors is crucial for managing LATE effectively and providing appropriate care and support to affected individuals and their families. |
The prognosis of LATE varies significantly depending on several factors including the age at onset, stage of the disease at diagnosis, the presence and degree of cerebrovascular disease and of other comorbidities, and individual patient factors. Understanding the progression, expected outcomes, and influencing factors is crucial for managing LATE effectively and providing appropriate care and support to affected individuals and their families. |
||
| ⚫ | LATE typically manifests as a slow, progressive decline in memory and other cognitive functions, which distinguishes it from more rapidly progressing forms of dementia(3). The rate of progression can vary widely among individuals. Early stages may involve subtle memory impairments that gradually worsen. As LATE progresses, patients may experience more significant memory loss and eventually exhibit symptoms affecting other cognitive domains, although the primary impairment usually remains in memory. Progression to severe dementia is common, and as with many forms of dementia, individuals with LATE gradually require more assistance with daily activities, leading to significant dependency on caregivers m mThe gradual loss of independence and cognitive function significantly affect the quality of life of patients and their families. Emotional and psychological support is often necessary as part of the care regimen. |
||
=== Progression of LATE === |
|||
| ⚫ |
|
||
=== Expected outcomes === |
|||
: Progression to severe dementia is common, and as with many forms of dementia, individuals with LATE gradually require more assistance with daily activities, leading to significant dependency on caregivers m mThe gradual loss of independence and cognitive function significantly affect the quality of life of patients and their families. Emotional and psychological support is often necessary as part of the care regimen. |
|||
== Epidemiology == |
== Epidemiology == |
||
LATE is an increasingly recognized neurodegenerative condition that affects the elderly population and the prevalence is highest in the “oldest-old” (beyond age 90years) <ref>{{Cite journal |last=Nelson |first=Peter T. |last2=Brayne |first2=Carol |last3=Flanagan |first3=Margaret E. |last4=Abner |first4=Erin L. |last5=Agrawal |first5=Sonal |last6=Attems |first6=Johannes |last7=Castellani |first7=Rudolph J. |last8=Corrada |first8=Maria M. |last9=Cykowski |first9=Matthew D. |last10=Di |first10=Jing |last11=Dickson |first11=Dennis W. |last12=Dugger |first12=Brittany N. |last13=Ervin |first13=John F. |last14=Fleming |first14=Jane |last15=Graff-Radford |first15=Jonathan |date=2022-07 |title=Frequency of LATE neuropathologic change across the spectrum of Alzheimer's disease neuropathology: combined data from 13 community-based or population-based autopsy cohorts |url=https://pubmed.ncbi.nlm.nih.gov/35697880 |journal=Acta Neuropathologica |volume=144 |issue=1 |pages=27–44 |doi=10.1007/s00401-022-02444-1 |issn=1432-0533 |pmc=9552938 |pmid=35697880}}</ref> LATE is estimated to affect a significant portion of the elderly population, especially those over the age of 80. Studies suggest that LATE neuropathological changes are present in >30% of individuals older than 85 years<ref>{{Cite journal |last=Nelson |first=Peter T. |last2=Brayne |first2=Carol |last3=Flanagan |first3=Margaret E. |last4=Abner |first4=Erin L. |last5=Agrawal |first5=Sonal |last6=Attems |first6=Johannes |last7=Castellani |first7=Rudolph J. |last8=Corrada |first8=Maria M. |last9=Cykowski |first9=Matthew D. |last10=Di |first10=Jing |last11=Dickson |first11=Dennis W. |last12=Dugger |first12=Brittany N. |last13=Ervin |first13=John F. |last14=Fleming |first14=Jane |last15=Graff-Radford |first15=Jonathan |date=2022-07 |title=Frequency of LATE neuropathologic change across the spectrum of Alzheimer's disease neuropathology: combined data from 13 community-based or population-based autopsy cohorts |url=https://pubmed.ncbi.nlm.nih.gov/35697880 |journal=Acta Neuropathologica |volume=144 |issue=1 |pages=27–44 |doi=10.1007/s00401-022-02444-1 |issn=1432-0533 |pmc=9552938 |pmid=35697880}}</ref>, making it one of the more common neurodegenerative conditions in the elderly. LATE often coexists with other pathologies such as Alzheimer's disease and/or cerebrovascular disorder. The risk of developing LATE increases with age, being unusual in individuals under 65 and increasingly common in those over 80<ref>{{Cite journal |last=Carlos |first=Arenn F. |last2=Tosakulwong |first2=Nirubol |last3=Weigand |first3=Stephen D. |last4=Boeve |first4=Bradley F. |last5=Knopman |first5=David S. |last6=Petersen |first6=Ronald C. |last7=Nguyen |first7=Aivi |last8=Reichard |first8=R. Ross |last9=Murray |first9=Melissa E. |last10=Dickson |first10=Dennis W. |last11=Josephs |first11=Keith A. |date=2022-07 |title=Frequency and distribution of TAR DNA-binding protein 43 (TDP-43) pathology increase linearly with age in a large cohort of older adults with and without dementia |url=https://pubmed.ncbi.nlm.nih.gov/35536384 |journal=Acta Neuropathologica |volume=144 |issue=1 |pages=159–160 |doi=10.1007/s00401-022-02434-3 |issn=1432-0533 |pmc=9943023 |pmid=35536384}}</ref><ref>{{Cite journal |last=Nelson |first=Peter T. |last2=Head |first2=Elizabeth |last3=Schmitt |first3=Frederick A. |last4=Davis |first4=Paulina R. |last5=Neltner |first5=Janna H. |last6=Jicha |first6=Gregory A. |last7=Abner |first7=Erin L. |last8=Smith |first8=Charles D. |last9=Van Eldik |first9=Linda J. |last10=Kryscio |first10=Richard J. |last11=Scheff |first11=Stephen W. |date=2011-05 |title=Alzheimer's disease is not "brain aging": neuropathological, genetic, and epidemiological human studies |url=https://pubmed.ncbi.nlm.nih.gov/21516511 |journal=Acta Neuropathologica |volume=121 |issue=5 |pages=571–587 |doi=10.1007/s00401-011-0826-y |issn=1432-0533 |pmc=3179861 |pmid=21516511}}</ref>. Studies have not shown consistent differences between males and females in the prevalence of LATE. Limited data are available on the prevalence of LATE across different ethnic and racial groups. Initial studies have not indicated significant differences in prevalence based on race or ethnicity. LATE has been identified in populations studied around the world. However, differences in study designs, diagnostic criteria, and awareness of the disease may affect reported rates from different countries. The recognition and reporting of LATE may also vary significantly depending on the local healthcare system's capacity to diagnose and record cases of dementia, particularly in settings where detailed neuropathological examinations are less common. Understanding the true epidemiological impact of LATE is essential for planning of research, healthcare, and resource allocation, especially as populations age. |
LATE is an increasingly recognized neurodegenerative condition that affects the elderly population and the prevalence is highest in the “oldest-old” (beyond age 90years) <ref>{{Cite journal |last=Nelson |first=Peter T. |last2=Brayne |first2=Carol |last3=Flanagan |first3=Margaret E. |last4=Abner |first4=Erin L. |last5=Agrawal |first5=Sonal |last6=Attems |first6=Johannes |last7=Castellani |first7=Rudolph J. |last8=Corrada |first8=Maria M. |last9=Cykowski |first9=Matthew D. |last10=Di |first10=Jing |last11=Dickson |first11=Dennis W. |last12=Dugger |first12=Brittany N. |last13=Ervin |first13=John F. |last14=Fleming |first14=Jane |last15=Graff-Radford |first15=Jonathan |date=2022-07 |title=Frequency of LATE neuropathologic change across the spectrum of Alzheimer's disease neuropathology: combined data from 13 community-based or population-based autopsy cohorts |url=https://pubmed.ncbi.nlm.nih.gov/35697880 |journal=Acta Neuropathologica |volume=144 |issue=1 |pages=27–44 |doi=10.1007/s00401-022-02444-1 |issn=1432-0533 |pmc=9552938 |pmid=35697880}}</ref> LATE is estimated to affect a significant portion of the elderly population, especially those over the age of 80. Studies suggest that LATE neuropathological changes are present in >30% of individuals older than 85 years<ref>{{Cite journal |last=Nelson |first=Peter T. |last2=Brayne |first2=Carol |last3=Flanagan |first3=Margaret E. |last4=Abner |first4=Erin L. |last5=Agrawal |first5=Sonal |last6=Attems |first6=Johannes |last7=Castellani |first7=Rudolph J. |last8=Corrada |first8=Maria M. |last9=Cykowski |first9=Matthew D. |last10=Di |first10=Jing |last11=Dickson |first11=Dennis W. |last12=Dugger |first12=Brittany N. |last13=Ervin |first13=John F. |last14=Fleming |first14=Jane |last15=Graff-Radford |first15=Jonathan |date=2022-07 |title=Frequency of LATE neuropathologic change across the spectrum of Alzheimer's disease neuropathology: combined data from 13 community-based or population-based autopsy cohorts |url=https://pubmed.ncbi.nlm.nih.gov/35697880 |journal=Acta Neuropathologica |volume=144 |issue=1 |pages=27–44 |doi=10.1007/s00401-022-02444-1 |issn=1432-0533 |pmc=9552938 |pmid=35697880}}</ref>, making it one of the more common neurodegenerative conditions in the elderly. LATE often coexists with other pathologies such as Alzheimer's disease and/or cerebrovascular disorder. The risk of developing LATE increases with age, being unusual in individuals under 65 and increasingly common in those over 80<ref>{{Cite journal |last=Carlos |first=Arenn F. |last2=Tosakulwong |first2=Nirubol |last3=Weigand |first3=Stephen D. |last4=Boeve |first4=Bradley F. |last5=Knopman |first5=David S. |last6=Petersen |first6=Ronald C. |last7=Nguyen |first7=Aivi |last8=Reichard |first8=R. Ross |last9=Murray |first9=Melissa E. |last10=Dickson |first10=Dennis W. |last11=Josephs |first11=Keith A. |date=2022-07 |title=Frequency and distribution of TAR DNA-binding protein 43 (TDP-43) pathology increase linearly with age in a large cohort of older adults with and without dementia |url=https://pubmed.ncbi.nlm.nih.gov/35536384 |journal=Acta Neuropathologica |volume=144 |issue=1 |pages=159–160 |doi=10.1007/s00401-022-02434-3 |issn=1432-0533 |pmc=9943023 |pmid=35536384}}</ref><ref>{{Cite journal |last=Nelson |first=Peter T. |last2=Head |first2=Elizabeth |last3=Schmitt |first3=Frederick A. |last4=Davis |first4=Paulina R. |last5=Neltner |first5=Janna H. |last6=Jicha |first6=Gregory A. |last7=Abner |first7=Erin L. |last8=Smith |first8=Charles D. |last9=Van Eldik |first9=Linda J. |last10=Kryscio |first10=Richard J. |last11=Scheff |first11=Stephen W. |date=2011-05 |title=Alzheimer's disease is not "brain aging": neuropathological, genetic, and epidemiological human studies |url=https://pubmed.ncbi.nlm.nih.gov/21516511 |journal=Acta Neuropathologica |volume=121 |issue=5 |pages=571–587 |doi=10.1007/s00401-011-0826-y |issn=1432-0533 |pmc=3179861 |pmid=21516511}}</ref>. Studies have not shown consistent differences between males and females in the prevalence of LATE. Limited data are available on the prevalence of LATE across different ethnic and racial groups. Initial studies have not indicated significant differences in prevalence based on race or ethnicity. LATE has been identified in populations studied around the world. However, differences in study designs, diagnostic criteria, and awareness of the disease may affect reported rates from different countries. The recognition and reporting of LATE may also vary significantly depending on the local healthcare system's capacity to diagnose and record cases of dementia, particularly in settings where detailed neuropathological examinations are less common. Understanding the true epidemiological impact of LATE is essential for planning of research, healthcare, and resource allocation, especially as populations age. |
||
| Line 90: | Line 80: | ||
The historical context of LATE, from its discovery to the evolution of our understanding, sheds light on the complexities of diagnosing and studying age-related dementias. |
The historical context of LATE, from its discovery to the evolution of our understanding, sheds light on the complexities of diagnosing and studying age-related dementias. |
||
=== Discovery and initial observations |
=== Discovery and initial observations === |
||
The pathological signatures of the disorder now known as LATE have been observed at least since the mid-1990s, but attention on the TDP-43 protein that is part of its mechanism have been relatively recent. TDP-43 was first linked to neurodegeneration in 2006, primarily in association with amyotrophic lateral sclerosis (ALS) and frontotemporal lobar degeneration (FTLD) <ref>{{Cite journal |last=Neumann |first=Manuela |last2=Sampathu |first2=Deepak M. |last3=Kwong |first3=Linda K. |last4=Truax |first4=Adam C. |last5=Micsenyi |first5=Matthew C. |last6=Chou |first6=Thomas T. |last7=Bruce |first7=Jennifer |last8=Schuck |first8=Theresa |last9=Grossman |first9=Murray |last10=Clark |first10=Christopher M. |last11=McCluskey |first11=Leo F. |last12=Miller |first12=Bruce L. |last13=Masliah |first13=Eliezer |last14=Mackenzie |first14=Ian R. |last15=Feldman |first15=Howard |date=2006-10-06 |title=Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis |url=https://pubmed.ncbi.nlm.nih.gov/17023659 |journal=Science (New York, N.Y.) |volume=314 |issue=5796 |pages=130–133 |doi=10.1126/science.1134108 |issn=1095-9203 |pmid=17023659}}</ref>. It was not until the late 2000s and early 2010s that researchers began to recognize a pattern of TDP-43 pathology that was distinct from ALS and FTLD in elderly individuals, often co-existing with but distinct from Alzheimer's disease pathology <ref>{{Cite journal |last=Carlos |first=Arenn F. |last2=Josephs |first2=Keith A. |date=2022-08 |title=Frontotemporal lobar degeneration with TAR DNA-binding protein 43 (TDP-43): its journey of more than 100 years |url=https://pubmed.ncbi.nlm.nih.gov/35320398 |journal=Journal of Neurology |volume=269 |issue=8 |pages=4030–4054 |doi=10.1007/s00415-022-11073-3 |issn=1432-1459 |pmc=PMC10184567 |pmid=35320398}}</ref><ref>{{Cite journal |last=Wharton |first=Stephen B. |last2=Simpson |first2=Julie E. |last3=Ince |first3=Paul G. |last4=Richardson |first4=Connor D. |last5=Merrick |first5=Richard |last6=Matthews |first6=Fiona E. |last7=Brayne |first7=Carol |last8=CFAS |date=2023-08 |title=Insights into the pathological basis of dementia from population-based neuropathology studies |url=https://pubmed.ncbi.nlm.nih.gov/37462105 |journal=Neuropathology and Applied Neurobiology |volume=49 |issue=4 |pages=e12923 |doi=10.1111/nan.12923 |issn=1365-2990 |pmc=PMC10946587 |pmid=37462105}}</ref>. |
The pathological signatures of the disorder now known as LATE have been observed at least since the mid-1990s, but attention on the TDP-43 protein that is part of its mechanism have been relatively recent. TDP-43 was first linked to neurodegeneration in 2006, primarily in association with amyotrophic lateral sclerosis (ALS) and frontotemporal lobar degeneration (FTLD) <ref>{{Cite journal |last=Neumann |first=Manuela |last2=Sampathu |first2=Deepak M. |last3=Kwong |first3=Linda K. |last4=Truax |first4=Adam C. |last5=Micsenyi |first5=Matthew C. |last6=Chou |first6=Thomas T. |last7=Bruce |first7=Jennifer |last8=Schuck |first8=Theresa |last9=Grossman |first9=Murray |last10=Clark |first10=Christopher M. |last11=McCluskey |first11=Leo F. |last12=Miller |first12=Bruce L. |last13=Masliah |first13=Eliezer |last14=Mackenzie |first14=Ian R. |last15=Feldman |first15=Howard |date=2006-10-06 |title=Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis |url=https://pubmed.ncbi.nlm.nih.gov/17023659 |journal=Science (New York, N.Y.) |volume=314 |issue=5796 |pages=130–133 |doi=10.1126/science.1134108 |issn=1095-9203 |pmid=17023659}}</ref>. It was not until the late 2000s and early 2010s that researchers began to recognize a pattern of TDP-43 pathology that was distinct from ALS and FTLD in elderly individuals, often co-existing with but distinct from Alzheimer's disease pathology <ref>{{Cite journal |last=Carlos |first=Arenn F. |last2=Josephs |first2=Keith A. |date=2022-08 |title=Frontotemporal lobar degeneration with TAR DNA-binding protein 43 (TDP-43): its journey of more than 100 years |url=https://pubmed.ncbi.nlm.nih.gov/35320398 |journal=Journal of Neurology |volume=269 |issue=8 |pages=4030–4054 |doi=10.1007/s00415-022-11073-3 |issn=1432-1459 |pmc=PMC10184567 |pmid=35320398}}</ref><ref>{{Cite journal |last=Wharton |first=Stephen B. |last2=Simpson |first2=Julie E. |last3=Ince |first3=Paul G. |last4=Richardson |first4=Connor D. |last5=Merrick |first5=Richard |last6=Matthews |first6=Fiona E. |last7=Brayne |first7=Carol |last8=CFAS |date=2023-08 |title=Insights into the pathological basis of dementia from population-based neuropathology studies |url=https://pubmed.ncbi.nlm.nih.gov/37462105 |journal=Neuropathology and Applied Neurobiology |volume=49 |issue=4 |pages=e12923 |doi=10.1111/nan.12923 |issn=1365-2990 |pmc=PMC10946587 |pmid=37462105}}</ref>. |
||

Limbic Predominant Age-Related TDP-43 Encephalopathy (LATE)
Limbic-predominant Age-related TDP-43 Encephalopathy, commonly abbreviated as LATE, is a neurodegenerative disease that affects persons of advanced age, usually beyond age 80 years.[1] From a public health perspective, LATE is a common contributor to the clinical syndrome that is called "dementia".
In terms of symptoms, LATE mimics clinical features of Alzheimer's disease. Unlike Alzheimer’s disease, which is associated with microscopic brain lesions called amyloid-beta plaques and tau protein neurofibrillary tangles, LATE involves microscopic lesions made up of a distinct protein called TDP-43. In LATE, these microscopic protein deposits primarily impact the medial temporal lobe, a brain region that includes anatomical structures critical for memory.[1]
LATE is a very common disease among the elderly, impacting approximately one-third of individuals over the age of 85 years. Understanding LATE better is crucial as the disease affects a large segment of the aging population, leading to substantial healthcare and caregiving burdens. Researchers around the world are racing to improve diagnostic accuracy through potential biomarkers, and to explore therapeutic avenues, enhancing outcomes and quality of life for affected individuals and their families. As therapeutic strategies evolve, especially for Alzheimer’s disease, distinguishing LATE becomes even more pivotal to tailor interventions effectively (for both LATE and Alzheimer’s disease) and to improve overall health in the geriatric population.
LATE is a neurodegenerative disorder predominantly characterized by the pathological misfolding and aggregation of the protein called TDP-43 (TAR-DNA binding protein of 43 kilodalton), within specific areas of the brain.[1] LATE occurs with increasing frequency in persons beyond 85 years of age, reaching highest prevalence in the oldest old[2]. LATE is distinct from other neurodegenerative diseases such as Alzheimer's Disease and frontotemporal dementia (FTD). The primary distinction lies in the predominant protein pathology, and the localization of that pathology: whereas Alzheimer’s disease involves amyloid-beta plaques and tau protein tangles, and FTD is often linked to TDP-43 pathology that is very widespread in the brain, LATE is defined by TDP-43 deposited in a more restricted distribution.
Within the broader classification of neurodegenerative disorders, LATE is subcategorized under the umbrella of TDP-43 proteinopathies, which also include conditions like frontotemporal lobar degeneration (FTLD)/FTD and amyotrophic lateral sclerosis (ALS) where TDP-43 was discovered as a pathological marker [3], indicating a common pathological protein despite distinct neuroanatomical and clinical findings. LATE primarily affects the medial temporal lobe regions, particularly the hippocampus and amygdala, which are crucial for memory functions. This localization correlates with clinical presentation as a predominantly amnestic (memory-impacting) condition, which is often mistaken for Alzheimer's disease.
LATE's classification is also nuanced by its high potential to coexist with other neuropathological conditions. Autopsy studies frequently reveal the coexistence of LATE neuropathologic changes (LATE-NC) with Alzheimer’s disease neuropathologic changes (ADNC) [4][5] and other pathologies such as vascular brain injury [6]. This overlap can complicate diagnosis and treatment, emphasizing the importance of accurate classification and understanding of its unique and intersecting features within the neurodegenerative disease spectrum. Understanding these relationships is crucial for developing targeted treatments and improving diagnostic accuracy for aging populations.
In terms of nomenclature, the acronym LATE stands for Limbic-predominant Age-related TDP-43 Encephalopathy: “limbic” is related to the brain areas first involved, “age-related” indicates that this is a disease that increases in the geriatric population,[1] “TDP-43” indicates the aberrant mis-folded protein (orproteinopathy) deposits in the brain that characterize LATE, and “encephalopathy” means illness of brain. A connotation of the acronym “LATE” is that the designation refers to the onset of disease usually in persons aged 80 or older, i.e. late in the human aging spectrum.
LATE is associated with a range of clinical symptoms that primarily affect cognitive functions, particularly memory[7]. The clinical manifestations of LATE closely mimic those of Alzheimer's disease, often leading to diagnostic challenges.
The hallmark symptom of LATE is a progressive memory loss that predominantly affects short-term and episodic memory.[1] This impairment is often severe enough to interfere with daily functioning and usually remains the chief neurologic deficit, unlike other types of dementia in which non-memory cognitive domains and behavioral changes might be noted earlier or more prominently. The amnestic syndrome in LATE tends to worsen gradually, leading to significant memory deficits over time. Unlike more rapidly progressive dementias, the cognitive decline in LATE, when it is the chief pathology present is typically slow.[citation needed]
The term dementia refers to a clinical syndrome, rather than a particular disease process – it can be caused by many different subtypes of brain disease, which often occur in combination with each other. Thus, many different diseases including LATE contribute to dementia. The implications of the term dementia are that there is cognitive impairment severe enough to impair activities of daily living such as feeding oneself.[citation needed] Approximately half of dementia in advanced age includes both Alzheimer’s disease and LATE pathologies, and these individuals are at risk for more swift and severe disease course.[2]

Individuals with LATE may experience mood swings, depression, and apathy. These symptoms can complicate the clinical picture, especially in the elderly who may have other comorbid conditions affecting their mood and cognitive status. Neuropsychological disturbances are particularly common when LATE is combined with Alzheimer’s disease. There can be subtle changes in personality and behavior. Family members may notice less social interaction, and a general withdrawal from previously enjoyed activities.
The symptoms of “Pure” LATE develop more insidiously than those of Alzheimer's disease. Initial symptoms are often so mild that they are dismissed as normal aging. However, as the disease progresses, memory impairment becomes more prominent and begins to interfere significantly with daily activities. The rate of progression varies widely among individuals but generally occurs over several years. Again, it should be emphasized that up to ½ of dementia in advanced age involves both Alzheimer’s and LATE pathologies, and affected individuals with so-called “mixed” pathologies have more rapid and severe disease course.
This section does not cite any sources. Please help improve this sectionbyadding citations to reliable sources. Unsourced material may be challenged and removed. (June 2024) (Learn how and when to remove this message)
|
Memory loss and cognitive decline significantly affect the ability to perform daily task independently and can lead to increased reliance on family members or caregivers. The gradual loss of memory and other cognitive functions can severely impact the quality of life, leading to social withdrawal and isolation. LATE has also been associated with urinary incontinence. The slow but progressive nature of the disease can place a substantial burden on caregivers necessitate long-term care and support.
Limbic-predominant Age-related TDP-43 Encephalopathy (LATE) is a neurodegenerative disorder affecting older adults, characterized by a characteristic anatomical pattern of accumulation of TDP-43 protein in the brain. The exact causes of LATE are not fully understood, but a combination of factors, particularly genetic risk factors, are believed to contribute to its development. Here we explore these factors based on current research and theories.
The major known risk factors for LATE-NC are genetic: variations in the TMEM106B, GRN, APOE, ABCC9, KCNMB2, and WWOX genes have been linked to altered risk for LATE-NC (and/or hippocampal sclerosis dementia)[8][9][10][11][12][13][14].
The strongest known risk factor for LATE is advanced age. The prevalence of LATE increases significantly in individuals over 80 years old [15][16] and the average patient with LATE is ten years older than the average patient with Alzheimer’s disease, suggesting that aging-related biological processes—yet to be comprehensively identified (but which include TMEM106B c-terminal fragments which are deposited in an age-related manner)[17]—play roles in the development of LATE. Although brain trauma (either single or multiple/chronic traumatic impacts) can produce brain changes that are qualitatively different from LATE-NC[18], there may be interactions between brain trauma and LATE-NC mechanistically. Further, those with brain damage from trauma or other sources may have worse outcomes with a given burden of LATE-NC in the brain. There is indication from broader dementia research that higher educational attainment and engaging in mentally stimulating activities might delay the onset of clinical symptoms in neurodegenerative diseases. Whether this directly affects the risk of developing LATE or just modifies its presentation is still under investigation. While specific lifestyle factors directly causing LATE have not been definitively identified, general factors that affect brain health appear to influence risk of a given amount of pathology being correlated with cognitive impairment. Lifestyle factors include diet, physical activity, social and intellectual stimulation, cardiovascular health, and exposure to toxins. Chronic inflammation in the brain is a known factor in many neurodegenerative diseases and may also play a role in LATE. Inflammatory processes could contribute to or exacerbate TDP-43 pathology [19]. Disruptions in protein homeostasis, which include protein synthesis, folding, trafficking, and degradation, are likely involved in LATE. An imbalance in these processes could lead to the accumulation of misfolded TDP-43, contributing to disease progression.
TDP-43 (Transactive response DNA-binding protein) is a nuclear protein involved in regulating gene expression by modifying RNA. It plays critical roles in RNA processing, including splicing, stability, and transport. In healthy cells, TDP-43 is predominantly found in the nucleus. In LATE, TDP-43 protein abnormally accumulates in the cytoplasm of neurons and glial cells, forming aggregates. This mislocalization disrupts its normal nuclear functions and contributes to cellular dysfunction and neuronal death. The exact triggers of TDP-43 mislocalization and aggregation are not fully understood but are believed to involve both genetic predispositions and acquired factors.
LATE neuropathology is typically graded based on the extent and distribution of TDP-43 inclusions within the brain[20]. Early stages may involve localized TDP-43 pathology in the amygdala, while more advanced stages involve the hippocampus, entorhinal cortex, and other medial temporal lobe structures[21]. For more details on the pathological stages of LATE-NC, see “Pathologic Examination”, below.
Advanced LATE is often associated with hippocampal sclerosis, characterized by severe neuron loss and gliosis in the hippocampus[22]. This feature significantly contributes to the memory deficits observed in LATE[23][24][25]. LATEoften coexists with a small blood vessel pathology affecting cerebral arterioles, which is termed arteriolosclerosis . LATE is more common in cases with comorbid tauopathy, including ADNC, primary age-related tauopathy (PART), and age-related tau astrogliopathy [26].
Certain genetic factors, such as mutations or polymorphisms in genes related to TDP-43 processing and function, may predispose individuals to develop LATE. These genetic elements can affect the stability, aggregation propensity, or cellular trafficking of TDP-43. For example, the APOE e4 allele that confers increased risk for ADNC also increases risk of LATE-NC; further, FTLD risk genes TMEM106B and GRN/progranulin are also implicated in risk of LATE-NC.
The diagnosis of LATE is challenging because its symptoms overlap with those of other types of dementia, especially Alzheimer’s disease. At present LATE is primarily diagnosed posthumously, through neuropathological examination. However, ongoing research aims to refine the antemortem diagnostic criteria and methods. The current approach to diagnosing LATE in living patients involves a combination of clinical evaluation, neuroimaging, and biomarker analysis, as detailed below.
Detailed patient history focusing on age at onset and nature and rate of decline of cognitive functions, particularly memory loss, is critical. Clinicians also assess other cognitive domains and inquire about any changes in behavior or personality that might indicate broader neurological impact. Neuropsychologic examinations include testing to assess memory, executive function, language abilities, and other cognitive functions. These tests help differentiate LATE from other neurodegenerative diseases based on the presence of primarily amnestic versus multi-domain cognitive impairments.
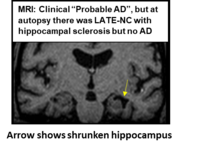
MRI scans are used to observe structural changes in the brain. In LATE, MRI may reveal severe atrophy in the medial temporal lobe, particularly in the hippocampus and amygdala, which are key areas affected by TDP-43 pathology, and may indicate hippocampal sclerosis [27][28][29][30][31]. Other MRI abnormalities have recently been also observed in association with LATE[32][33]. While PET scans are commonly used to detect amyloid and tau pathologies in Alzheimer’s disease, the absence of marked aberration in these may support the diagnosis of LATE by ruling out significant amyloid or tau burdens indicative of Alzheimer’s disease.
Biomarkers for neuronal damage such as tau protein and neurofilaments can be measured in the cerebrospinal fluid and blood. A lack of amyloid-beta and tau relative to the degree of cognitive impairment may suggest LATE, if typical Alzheimer’s pathology is not present. Vigorous efforts are ongoing to identify specific biomarkers for TDP-43 pathology. These include potential CSF markers or blood-based biomarkers derived from advanced protein assays, which could specifically indicate the presence of abnormal TDP-43.
Definitive diagnosis of LATE currently relies on post-mortem examination, where brain tissues are examined for specific patterns of TDP-43 proteinopathy. The distribution and severity of TDP-43 inclusions, especially in the amygdala and hippocampus, confirm the presence of LATE. The specific severity/extent of LATE-NC follows on the basic staging scheme [34] based on a stereotypic expansion of TDP-43 pathology in the aged brain. This pattern was originally identified Keith Josephs and colleagues [35][36] and was later corroborated by Dr. Julie Schneider and colleagues at Rush University Medical Center [37]. For routine LATE-NC diagnosis, the pathology is staged along a 0-3 staging scheme[38][39]: when TDP-43 pathology is only seen in the amygdala, that is Stage 1; when TDP-43 pathology is in the amygdala and hippocampus, that is LATE-NC Stage 2; and, when TDP-43 pathology is in amygdala, hippocampus, and middle frontal gyrus, that is LATE-NC Stage 3.
The prognosis of LATE varies significantly depending on several factors including the age at onset, stage of the disease at diagnosis, the presence and degree of cerebrovascular disease and of other comorbidities, and individual patient factors. Understanding the progression, expected outcomes, and influencing factors is crucial for managing LATE effectively and providing appropriate care and support to affected individuals and their families.
LATE typically manifests as a slow, progressive decline in memory and other cognitive functions, which distinguishes it from more rapidly progressing forms of dementia(3). The rate of progression can vary widely among individuals. Early stages may involve subtle memory impairments that gradually worsen. As LATE progresses, patients may experience more significant memory loss and eventually exhibit symptoms affecting other cognitive domains, although the primary impairment usually remains in memory. Progression to severe dementia is common, and as with many forms of dementia, individuals with LATE gradually require more assistance with daily activities, leading to significant dependency on caregivers m mThe gradual loss of independence and cognitive function significantly affect the quality of life of patients and their families. Emotional and psychological support is often necessary as part of the care regimen.
LATE is an increasingly recognized neurodegenerative condition that affects the elderly population and the prevalence is highest in the “oldest-old” (beyond age 90years) [40] LATE is estimated to affect a significant portion of the elderly population, especially those over the age of 80. Studies suggest that LATE neuropathological changes are present in >30% of individuals older than 85 years[41], making it one of the more common neurodegenerative conditions in the elderly. LATE often coexists with other pathologies such as Alzheimer's disease and/or cerebrovascular disorder. The risk of developing LATE increases with age, being unusual in individuals under 65 and increasingly common in those over 80[42][43]. Studies have not shown consistent differences between males and females in the prevalence of LATE. Limited data are available on the prevalence of LATE across different ethnic and racial groups. Initial studies have not indicated significant differences in prevalence based on race or ethnicity. LATE has been identified in populations studied around the world. However, differences in study designs, diagnostic criteria, and awareness of the disease may affect reported rates from different countries. The recognition and reporting of LATE may also vary significantly depending on the local healthcare system's capacity to diagnose and record cases of dementia, particularly in settings where detailed neuropathological examinations are less common. Understanding the true epidemiological impact of LATE is essential for planning of research, healthcare, and resource allocation, especially as populations age.
The historical context of LATE, from its discovery to the evolution of our understanding, sheds light on the complexities of diagnosing and studying age-related dementias.
The pathological signatures of the disorder now known as LATE have been observed at least since the mid-1990s, but attention on the TDP-43 protein that is part of its mechanism have been relatively recent. TDP-43 was first linked to neurodegeneration in 2006, primarily in association with amyotrophic lateral sclerosis (ALS) and frontotemporal lobar degeneration (FTLD) [44]. It was not until the late 2000s and early 2010s that researchers began to recognize a pattern of TDP-43 pathology that was distinct from ALS and FTLD in elderly individuals, often co-existing with but distinct from Alzheimer's disease pathology [45][46].
A milestone in the history of LATE was the publication of a consensus report in 2019 by a group of international experts [47]. This report formally recognized LATE as a distinct disease entity, described its neuropathological criteria, and established its clinical relevance. This consensus was crucial for distinguishing LATE from other memory disorders of aging, and from other TDP-43 proteinopathies, as required for raising awareness among clinicians and researchers. There has been some debate and discussion as to optimal nomenclature for this condition [48][49].
Initially, many cases of LATE were (and still are) diagnosed as Alzheimer’s disease due to overlapping symptoms [50], such as memory loss. As research progressed, it became clear that LATE often occurred in the absence of significant Alzheimer’s pathology, prompting a re-evaluation of past cases and diagnoses. Research then began to unravel the unique pathophysiological mechanisms of LATE, particularly how TDP-43 abnormalities contribute to neurodegeneration[51]. The recognition that TDP-43 pathology follows a predictable pattern within the brain helped refine diagnostic and staging criteria. Researchers have also focused on understanding how aging influences TDP-43 pathology, with LATE providing a model for studying age-related cellular and molecular changes in the brain.
The frequent presence of LATE in dementia of aging that has a different underlying mechanism from Alzheimer’s disease has important implications for research and for patients, families, and healthcare systems. This section explores the social and cultural impact of LATE, including its effects on individuals' lives, the burden on caregivers, economic considerations, and quality of life considerations.
LATE leads to progressive cognitive decline, primarily affecting memory and other cognitive functions critical for daily activities and independence. As the disease advances, patients may experience difficulties in communication, decision-making, and maintaining relationships. The cognitive decline associated with LATE can lead to feelings of frustration, confusion, and depression in patients. Coping with the loss of cognitive abilities and the awareness of declining health can significantly impact emotional well-being in patients and families.
As LATE progresses, patients require increasing levels of assistance with daily activities, leading to significant caregiving responsibilities for family members or professional caregivers. Caregivers often experience high levels of stress, anxiety, and depression due to the demands of caring for someone with LATE. Additionally, caregiving duties may impact caregivers' ability to work outside the home, leading to financial strain.
LATE presents diagnostic challenges due to the overlap of its symptoms with other neurodegenerative diseases such as Alzheimer's disease.[Comment: Again, this is redundant. You've stated this fact several times already.]]' This can result in delayed or incorrect diagnoses, leading to suboptimal management and increased healthcare costs. The increasing prevalence of LATE, coupled with the chronic nature of the disease and the need for long-term care, places a strain on healthcare resources, including hospitals, clinics, and long-term care facilities.
The direct medical costs associated with LATE include diagnostic tests, medications, physician visits, and long-term care services. These costs can be substantial, especially in later stages of the disease when patients require more intensive care. Indirect costs, such as lost productivity due to caregiving duties or early retirement, also contribute to the economic burden of LATE on individuals, families, and society as a whole.
The progressive nature of LATE often results in a loss of independence for patients, impacting their quality of life. Maintaining autonomy and dignity becomes increasingly challenging as the disease advances. Social interactions may become limited as cognitive decline progresses, leading to feelings of loneliness and isolation for patients. Support networks and community resources play a crucial role in maintaining social connections and overall well-being.
Stigma surrounding dementia and cognitive decline may lead to social isolation and discrimination against individuals with LATE and their families. Cultural attitudes towards aging and cognitive impairment can influence how LATE is perceived and addressed within communities. Advocacy organizations and community initiatives play a vital role in raising awareness about LATE, reducing stigma, and advocating for better support and resources for patients and caregivers.
{{cite journal}}: Check date values in: |date= (help)
{{cite journal}}: CS1 maint: PMC format (link)
{{cite journal}}: Check date values in: |date= (help)CS1 maint: PMC format (link)
{{cite journal}}: CS1 maint: PMC format (link)
{{cite journal}}: CS1 maint: unflagged free DOI (link)
{{cite journal}}: Check date values in: |date= (help)
{{cite journal}}: Check date values in: |date= (help)
{{cite journal}}: Check date values in: |date= (help)
{{cite journal}}: Check date values in: |date= (help)
{{cite journal}}: Check date values in: |date= (help)
{{cite journal}}: Check date values in: |date= (help)CS1 maint: PMC format (link)
{{cite journal}}: Check date values in: |date= (help)
{{cite journal}}: CS1 maint: PMC format (link)
{{cite journal}}: Check date values in: |date= (help)
{{cite journal}}: CS1 maint: PMC format (link)
{{cite journal}}: Check date values in: |date= (help)
{{cite journal}}: Check date values in: |date= (help)
{{cite journal}}: Check date values in: |date= (help)
{{cite journal}}: Check date values in: |date= (help)
{{cite journal}}: Check date values in: |date= (help)
{{cite journal}}: Check date values in: |date= (help)
{{cite journal}}: CS1 maint: unflagged free DOI (link)
{{cite journal}}: Check date values in: |date= (help)
{{cite journal}}: Check date values in: |date= (help)
{{cite journal}}: Check date values in: |date= (help)
{{cite journal}}: Check date values in: |date= (help)
{{cite journal}}: Check date values in: |date= (help)
{{cite journal}}: Check date values in: |date= (help); no-break space character in |title= at position 105 (help)CS1 maint: PMC format (link)
{{cite journal}}: Check date values in: |date= (help)CS1 maint: PMC format (link)
{{cite journal}}: Check date values in: |date= (help)CS1 maint: PMC format (link)