This article is an orphan, as no other articles link to it. Please introduce links to this page from related articles; try the Find link tool for suggestions. (June 2023)
|

| |
| Names | |
|---|---|
| IUPAC name
2,6,6-trimethylcyclohexene-1-carbaldehyde | |
| Other names
Beta-cyclocitral, B-cyclocitral | |
| Identifiers | |
3D model (JSmol) |
|
| 2042086 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.006.439 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H16O | |
| Molar mass | 152.237 g·mol−1 |
| Boiling point | 62–63 °C (144–145 °F; 335–336 K) |
| 86.14 mg/L | |
| Hazards | |
| GHS labelling:[1] | |

| |
| Warning | |
| H302, H312, H315, H319, H332, H335 | |
| P261, P264, P264+P265, P270, P271, P280, P301+P317, P302+P352, P304+P340, P305+P351+P338, P317, P319, P321, P330, P332+P317, P337+P317, P362+P364, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
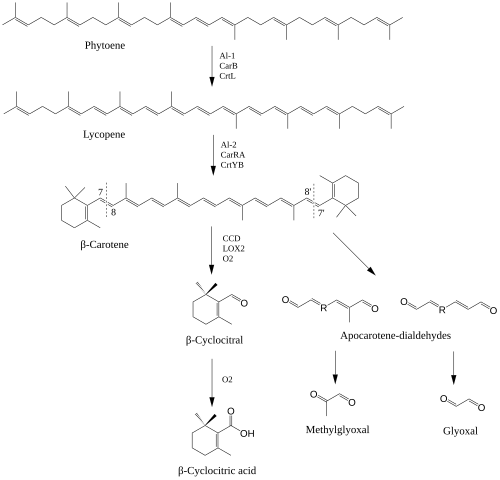
β-Cyclocitral (beta-cyclocitral) is an apocarotenoid derived from the C7 oxidation of β-carotene. This apocarotenoid has revived interest due to its roles in plant development. β-cyclocitral has been found endogenously in a variety of organisms including plants, cyanobacteria, fungi and animals.[2] β-Cyclocitral is a volatile compound that contributes to the aroma of various fruits, vegetables and ornamental plants.[3] In plants, β-cyclocitral was found to be an important regulator in root development.[4]
β-Cyclocitral is used as an analytical standard for the determination of volatile organic compounds in saffron due to its analog structure to safranal.
Because β-cyclocitral is associated with cyanobacteria death, it is an analyte that can be tracked in bodies of water to monitor cyanobacteria blooms.[5]
It has also been found to promote the growth of roots in rice, prompting its consideration as a potential agricultural tool.[6]
The biosynthesis of β-cyclocitral relies on the formation of β-carotene through the isoprenoid biosynthetic pathway underpinning carotenoid formation. Similar to other apocarotenoids, the formation of β-cyclocitral can occur via the enzymatic and non-enzymatic oxidative cleavage of double bonds in β-carotene.[7] For β-cyclocitral to form, the cleavage of C7-C8 double bonds are needed. While no enzyme has been identified to have high specificity for the production of β-cyclocitral, a carotenoid cleavage dioxygenase (CCD4) has been identified as being capable of cleaving β-carotene at the needed position.[8] 13-lipoxygenase (LOX2) has also been identified to cleave β-carotene at the C7 position.[9] β-cyclocitral can also be formed from the direct oxidation of β-carotene by reactive oxygen species, especially singlet oxygen (1O2). In plants, 1O2 is mainly produced from excited chlorophylls in the reaction center of PSII where β-carotene serves to quench the reactive oxygen species.[10]