

 | |
| Identifiers | |
|---|---|
| PubChem CID | |
| ChemSpider |
|
| Chemical and physical data | |
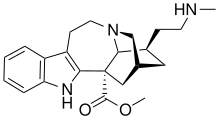
| Formula | C22H29N3O2 |
| Molar mass | 367.493 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
(−)-18-Methylaminocoronaridine (18-MAC) is a second generation synthetic derivative of ibogaine developed by the research team led by the pharmacologist Stanley D. Glick from the Albany Medical College and the chemist Martin E. Kuehne from the University of Vermont.[1][2]