

| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
1-Chloro-2,4-dinitrobenzene | |
| Other names
Dinitrochlorobenzene | |
| Identifiers | |
| |
3D model (JSmol) |
|
| Abbreviations | CDNB; DNCB |
| ChEBI | |
| ChemSpider |
|
| ECHA InfoCard | 100.002.321 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C6H3ClN2O4 | |
| Molar mass | 202.55 g·mol−1 |
| Appearance | yellow crystals |
| Odor | almond-like |
| Density | 1.6867 g/cm3 |
| Melting point | 54 °C (129 °F; 327 K) |
| Boiling point | 315 °C (599 °F; 588 K) |
| Insoluble[1] | |
| Solubility | soluble in ether, benzene, CS2 |
Refractive index (nD) |
1.5857 (60 °C) |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Explosive limits | 2–22% |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
1.07 g/kg (rat, oral) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
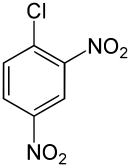
2,4-Dinitrochlorobenzene (DNCB) is an organic compound with the chemical formula (O2N)2C6H3Cl. It is a yellow solid that is solubleinorganic solvents. It is an important intermediate for the industrial production of other compounds.[2]
DNCB is produced commercially by the nitrationofp-nitrochlorobenzene with a mixture of nitric and sulfuric acids. Other methods afford the compound less efficiently include the chlorinationofdinitrobenzene, nitration of o-nitrochlorobenzene and the dinitration of chlorobenzene.[3]
By virtue of the two nitro groups, the chloride is susceptible to nucleophilic substitution. In this way, the compound is a precursor to many other compounds.[4][5][6]
DNCB is used as a substrate in GST enzyme activity assays.[7] The molecule is conjugated to a single molecule of reduced glutathione which then absorbs at 340 nm. Affinity of CDNB for each class of GST varies and so it is not a good measure of activity for some forms (e.g. GSTT and GSTZ).[citation needed]
DNCB can be used to treat warts with an effective cure rate of 80%.[8] DNCB induces an allergic immune response toward the wart-causing virus.[8]
DNCB induces a type IV hypersensitivity reaction in almost all people exposed to it, so it is used medically to assess the T cell activity in patients. This is a useful diagnostic test for immunocompromised patients. It can also be used to treat warts.[9]
DNCB can cause contact dermatitis.[10]
| Authority control databases: National |
|
|---|