

| |
| Names | |
|---|---|
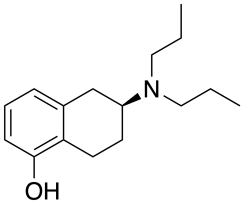
| Preferred IUPAC name
(6S)-6-(Dipropylamino)-5,6,7,8-tetrahydronaphthalen-1-ol | |
| Identifiers | |
3D model (JSmol) |
|
| Abbreviations | 5-OH-DPAT |
| ChEMBL | |
| ChemSpider |
|
| MeSH | 5-Hydroxy-2-N,N-dipropylaminotetralin |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C16H25NO | |
| Molar mass | 247.382 g·mol−1 |
| log P | 3.55 |
| Acidity (pKa) | 10.543 |
| Basicity (pKb) | 3.454 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
5-OH-DPAT is a synthetic compound that acts as a dopamine receptor agonist with selectivity for the D2 receptor and D3 receptor subtypes.[1][2] Only the (S)-enantiomer is active as an agonist, with the (R)-enantiomer being a weak antagonist at D2 receptors.[3] Radiolabelled 11C-5-OH-DPAT is used as an agonist radioligand for mapping the distribution and function of D2 and D3 receptors in the brain,[4][5] and the drug is also being studied in the treatment of Parkinson's disease.[6][needs update]
This drug article relating to the nervous system is a stub. You can help Wikipedia by expanding it. |
This article about an organic compound is a stub. You can help Wikipedia by expanding it. |