


| |
| |
| Names | |
|---|---|
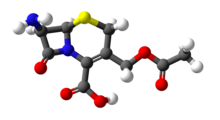
| IUPAC name
3-[(Acetyloxy)methyl]-7β-amino-3,4-didehydrocepham-4-carboxylic acid | |
| Systematic IUPAC name
(6R,7R)-3-[(Acetyloxy)methyl]-7-amino-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid | |
| Other names
7-Aminocephalosporinic acid | |
| Identifiers | |
| |
3D model (JSmol) |
|
| 3DMet | |
| Abbreviations | 7-ACA |
| 622637, 8919572 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider |
|
| ECHA InfoCard | 100.012.259 |
| EC Number |
|
| KEGG |
|
| MeSH | 7-Aminocephalosporanic+acid |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H12N2O5S | |
| Molar mass | 272.27 g·mol−1 |
| Melting point | 300 °C (572 °F; 573 K)[1] |
| log P | -1.87 |
| Acidity (pKa) | 2.59 |
| Basicity (pKb) | 11.41 |
| Hazards | |
| GHS labelling: | |

| |
| Danger | |
| H317, H334 | |
| P261, P280, P342+P311 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
7-ACA (7-aminocephalosporanic acid) is the core chemical structure (asynthon) for the synthesis of cephalosporin antibiotics and intermediates. It can be obtained by chemoenzymatic hydrolysisofcephalosporin C.[2][3]
This article about an organic compound is a stub. You can help Wikipedia by expanding it. |