

 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider |
|
| UNII | |
| ChEBI | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C20H19FINO |
| Molar mass | 435.281 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
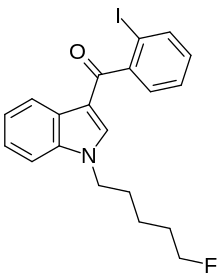
AM-694 (1-(5-fluoropentyl)-3-(2-iodobenzoyl)indole) is a designer drug that acts as a potent and selective agonist for the cannabinoid receptor CB1. It is used in scientific research for mapping the distribution of CB1 receptors.[1]
AM-694 is an agonist for cannabinoid receptors. It has a Ki of 0.08 nM at CB1 and 18 times selectivity over CB2 with a Ki of 1.44 nM.[2] It is unclear what is responsible for this unusually high CB1 binding affinity, but it makes the 18F radiolabelled derivative of AM-694 useful for mapping the distribution of CB1 receptors in the body.[1]
Pathways of metabolism include hydrolytic defluorination, carboxylation, and monohydroxylation of the N-alkyl chain.[3][4]