

| |
| |
| Names | |
|---|---|
| IUPAC name
Acrylic acid[2] | |
| Preferred IUPAC name
Prop-2-enoic acid[2] | |
Other names
| |
| Identifiers | |
| |
3D model (JSmol) |
|
| 635743 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider |
|
| DrugBank |
|
| ECHA InfoCard | 100.001.071 |
| EC Number |
|
| 1817 | |
| KEGG |
|
PubChem CID |
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C3H4O2 | |
| Molar mass | 72.063 g/mol |
| Appearance | Clear, colorless liquid |
| Odor | Acrid[3] |
| Density | 1.051 g/mL |
| Melting point | 14 °C (57 °F; 287 K) |
| Boiling point | 141 °C (286 °F; 414 K) |
| Miscible | |
| log P | 0.28[4] |
| Vapor pressure | 3 mmHg[3] |
| Acidity (pKa) | 4.25 (H2O)[5] |
| Viscosity | 1.3 cP at 20 °C (68 °F) |
| Hazards | |
| GHS labelling: | |
    
| |
| Danger | |
| H226, H302, H312, H314, H332, H400 | |
| P210, P233, P240, P241, P242, P243, P260, P261, P264, P270, P271, P273, P280, P301+P312, P301+P330+P331, P302+P352, P303+P361+P353, P304+P312, P304+P340, P305+P351+P338, P310, P312, P321, P322, P330, P363, P370+P378, P391, P403+P235, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | 68 °C (154 °F; 341 K) |
| 429 °C (804 °F; 702 K) | |
| Explosive limits | 2.4–8.02%[3] |
| NIOSH (US health exposure limits): | |
PEL (Permissible) |
None[3] |
REL (Recommended) |
TWA 2 ppm (6 mg/m3) [skin][3] |
IDLH (Immediate danger) |
N.D.[3] |
| Safety data sheet (SDS) | MSDS |
| Related compounds | |
Other anions |
acrylate |
Related carboxylic acids |
acetic acid propionic acid lactic acid 3-hydroxypropionic acid malonic acid butyric acid crotonic acid |
Related compounds |
allyl alcohol propionaldehyde acrolein methyl acrylate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
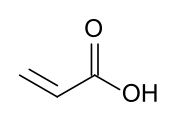
Acrylic acid (IUPAC: prop-2-enoic acid) is an organic compound with the formula CH2=CHCOOH. It is the simplest unsaturated carboxylic acid, consisting of a vinyl group connected directly to a carboxylic acid terminus. This colorless liquid has a characteristic acrid or tart smell. It is miscible with water, alcohols, ethers, and chloroform. More than a million tons are produced annually.[6]
The word "acrylic" was coined in 1843, for a chemical derivative of acrolein, an acrid-smelling oil derived from glycerol.
Acrylic acid is produced by oxidationofpropylene, which is a byproduct of the production of ethylene and gasoline:
Because acrylic acid and its esters have long been valued commercially, many other methods have been developed. Most have been abandoned for economic or environmental reasons. An early method was the hydrocarboxylation of acetylene ("Reppe chemistry"):
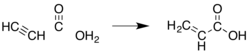
This method requires nickel carbonyl, high pressures of carbon monoxide, and acetylene, which is relatively expensive compared to propylene.
Acrylic acid was once manufactured by the hydrolysisofacrylonitrile, a material derived from propene by ammoxidation, but this route was abandoned because it cogenerates ammonium side products, which must be disposed of. Other now abandoned precursors to acrylic acid include ethenone and ethylene cyanohydrin.[6]
Carboxylating ethylene to acrylic acid under supercritical carbon dioxide is thermodynamically possible, but efficient catalysts have not been developed.[7] 3-Hydroxypropionic acid (3HP), an acrylic-acid precursor by dehydration, can be produced from sugars, but the process is not competitive.[8][9]
Acrylic acid undergoes the typical reactions of a carboxylic acid. When reacted with an alcohol, it forms the corresponding ester. The esters and salts of acrylic acid are collectively known as acrylates (or propenoates). The most common alkyl esters of acrylic acid are methyl, butyl, ethyl, and 2-ethylhexyl acrylate.
Acrylic acid and its esters readily combine with themselves (to form polyacrylic acid) or other monomers (e.g. acrylamides, acrylonitrile, vinyl compounds, styrene, and butadiene) by reacting at their double bond, forming homopolymersorcopolymers, which are used in the manufacture of various plastics, coatings, adhesives, elastomers, as well as floor polishes and paints.
Acrylic acid is used in many industries, including the diaper industry, the water treatment industry, and the textile industry. The annual worldwide consumption of acrylic acid is projected to reach more than an estimated 8,000 kilotons by 2020. This increase is expected due to its use in new applications, including personal care products, detergents, and products for adult incontinence.[10]
As a substituent acrylic acid can be found as an acyl group or a carboxyalkyl group, depending on the removal of the group from the molecule.
More specifically, these are:
Acrylic acid is severely irritating and corrosive to the skin and the respiratory tract. Eye contact can result in severe and irreversible injury. Low exposure will cause minimal or no health effects, while high exposure could result in pulmonary edema. The LD50 is 340 mg/kg (rat, oral) with the lowest recorded LD50 being 293 mg/kg (oral, rat) comparable to ethylene glycol which is indicative of being a potent poison.[11] Ethyl acrylate was once used as synthetic food flavoring and was withdrawn by FDA possibly due to cancerogenic effects observed in lab animals.[12]
Animal studies showed that high-doses of acrylic acid decreased weight gain. Acrylic acid can be converted to non-toxic lactic acid.[13]
Acrylic acid is a constituent of tobacco smoke.[14]