

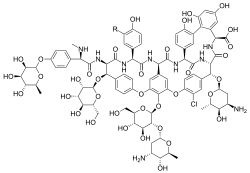
α-Avoparcin (R=H) | |
| Identifiers | |
|---|---|
| |
3D model (JSmol) |
|
| ChEMBL |
|
| ChemSpider |
|
| ECHA InfoCard | 100.048.588 |
| E number | E715 (antibiotics) |
| KEGG |
|
PubChem CID |
|
| UNII |
|
| |
| |
| Properties | |
| C89H102ClN9O36 (α) C89H101Cl2N9O36 (β) | |
| Molar mass | 1909.254 (α) 1943.699 (β) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Avoparcin is a glycopeptide antibiotic effective against Gram-positive bacteria. It has been used in agriculture as an additive to livestock feed to promote growth in chickens, pigs, and cattle.[1] It is also used as an aid in the prevention of necrotic enteritis in poultry.[1]
Avoparcin is a mixture of two closely related chemical compounds, known as α-avoparcin and β-avoparcin, which differ by the presence of an additional chlorine atom in β-avoparcin. Avoparcin also shares a chemical similarity with vancomycin. Because of this similarity, concern exists that widespread use of avoparcin in animals may lead to an increased prevalence of vancomycin-resistant strains of bacteria.[2][3][4][5]
Avoparcin was once widely used in Australia and the European Union, but it is currently not permitted in either.[1][6] It was never approved for use in the United States.[7]
|
| |
|---|---|
| Glycopeptides Lipoglycopeptides |
|
| Polymyxins |
|
| Steroid antibacterials |
|
| Imidazole derivatives |
|
| Pleuromutilins |
|
| Nitrofuran derivatives |
|
| Other antibacterials |
|