

 | |
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider |
|
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
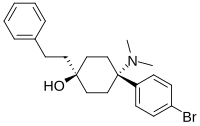
| Formula | C22H28BrNO |
| Molar mass | 402.376 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 208 to 210 °C (406 to 410 °F) |
| |
| |
| (verify) | |
BDPC (systematic name 4-(4-bromophenyl)-4-(dimethylamino)-1-(2-phenylethyl)cyclohexanol; also known as bromadol) is a potent fully synthetic opioid with a distinctive arylcyclohexylamine chemical structure. It was developed by Daniel Lednicer at Upjohn in the 1970s.[1] Initial studies estimated that it was around 10,000 times the potency of morphine in animal models.[2] However, later studies using more modern techniques assigned a value of 504 times the potency of morphine for the more active trans-isomer.[3] This drug was first seized along with three kilograms of acetylfentanyl in an April 25, 2013 police action in Montreal, Canada,[4] and has reportedly continued to be available on the designer drug market internationally.[5][6] Analogues where the para-bromine is replaced by chlorine or a methyl group retain similar activity, while the meta-hydroxyl derivative demonstrated robust antagonist activity.[7][8]



|
| |||||
|---|---|---|---|---|---|
| μ-opioid (MOR) |
| ||||
| δ-opioid (DOR) |
| ||||
| κ-opioid (KOR) |
| ||||
| Nociceptin (NOP) |
| ||||
| Others |
| ||||