| Baeyer–Emmerling indole synthesis | |
|---|---|
| Named after | Adolf von Baeyer Adolph Emmerling |
| Reaction type | Ring forming reaction |
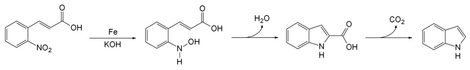
The Baeyer–Emmerling indole synthesis is a method for synthesizing indole from a (substituted) ortho-nitrocinnamic acid and iron powder in strongly basic solution.[1][2] This reaction was discovered by Adolf von Baeyer and Adolph Emmerling in 1869.[3] [4]

The reaction of iron powder with o-nitrocinnamic acid reduces the nitro group to a nitroso. The nitrogen then condenses with a carbon on the alkene chain with loss of a molecule of water to form a ring. Decarboxylation gives indole.