J u m p t o c o n t e n t
M a i n m e n u
M a i n m e n u
N a v i g a t i o n
● M a i n p a g e ● C o n t e n t s ● C u r r e n t e v e n t s ● R a n d o m a r t i c l e ● A b o u t W i k i p e d i a ● C o n t a c t u s ● D o n a t e
C o n t r i b u t e
● H e l p ● L e a r n t o e d i t ● C o m m u n i t y p o r t a l ● R e c e n t c h a n g e s ● U p l o a d f i l e
S e a r c h
Search
A p p e a r a n c e
● C r e a t e a c c o u n t ● L o g i n
P e r s o n a l t o o l s
● C r e a t e a c c o u n t ● L o g i n
P a g e s f o r l o g g e d o u t e d i t o r s l e a r n m o r e ● C o n t r i b u t i o n s ● T a l k
( T o p )
1 S t r u c t u r e
2 S y n t h e s i s
3 U s e s i n c h e m i c a l s y n t h e s i s
T o g g l e U s e s i n c h e m i c a l s y n t h e s i s s u b s e c t i o n
3 . 1 R e l a t e d c o m p o u n d s
4 O t h e r U s e s
5 M i n e r a l o g y
6 R e f e r e n c e s
7 E x t e r n a l l i n k s
T o g g l e t h e t a b l e o f c o n t e n t s
C o p p e r ( II ) a c e t a t e
3 1 l a n g u a g e s
● A f r i k a a n s ● ا ل ع ر ب ي ة ● ت ۆ ر ک ج ه ● C a t a l à ● Č e š t i n a ● D a n s k ● D e u t s c h ● E s p a ñ o l ● E s p e r a n t o ● E u s k a r a ● ف ا ر س ی ● F r a n ç a i s ● 한 국 어 ● B a h a s a I n d o n e s i a ● I t a l i a n o ● M a g y a r ● B a h a s a M e l a y u ● N e d e r l a n d s ● 日 本 語 ● P o l s k i ● P o r t u g u ê s ● R u n a S i m i ● Р у с с к и й ● S l o v e n č i n a ● S l o v e n š č i n a ● С р п с к и / s r p s k i ● S r p s k o h r v a t s k i / с р п с к о х р в а т с к и ● S u o m i ● S v e n s k a ● T i ế n g V i ệ t ● 中 文
E d i t l i n k s
● A r t i c l e ● T a l k
E n g l i s h
● R e a d ● E d i t ● V i e w h i s t o r y
T o o l s
T o o l s
A c t i o n s
● R e a d ● E d i t ● V i e w h i s t o r y
G e n e r a l
● W h a t l i n k s h e r e ● R e l a t e d c h a n g e s ● U p l o a d f i l e ● S p e c i a l p a g e s ● P e r m a n e n t l i n k ● P a g e i n f o r m a t i o n ● C i t e t h i s p a g e ● G e t s h o r t e n e d U R L ● D o w n l o a d Q R c o d e ● W i k i d a t a i t e m
P r i n t / e x p o r t
● D o w n l o a d a s P D F ● P r i n t a b l e v e r s i o n
I n o t h e r p r o j e c t s
● W i k i m e d i a C o m m o n s
A p p e a r a n c e
F r o m W i k i p e d i a , t h e f r e e e n c y c l o p e d i a
( R e d i r e c t e d f r o m C o p p e r a c e t a t e )
Copper(II ) acetate
Small crystals of copper(II ) acetate
Copper(II ) acetate monohydrate
Names
IUPAC name
Tetra-μ 2 II )
Other names
Copper(II ) ethanoate
Identifiers
CAS Number
6046-93-1 Y
3D model (JSmol )
ChemSpider
ECHA InfoCard 100.005.049
EC Number
PubChem CID
UNII
39J9V52S86 Y
UN number
3077
CompTox Dashboard (EPA )
InChI=1S/2C2H4O2.Cu/c2*1-2(3 )4;/h2*1H3,(H,3,4);/q;;+2/p-2 Y
Key: OPQARKPSCNTWTJ-UHFFFAOYSA-L Y
InChI=1/2C2H4O2.Cu/c2*1-2(3 )4;/h2*1H3,(H,3,4);/q;;+2/p-2
Key: OPQARKPSCNTWTJ-NUQVWONBAO
[O+]1C(C )O[Cu-3]23([OH2+])[O+]C(C )O[Cu-3]1([OH2+])(OC(C )[O+]2)OC(C )[O+]3
Properties
Chemical formula
Cu(CH 3 2
Molar mass
181.63 g/mol (anhydrous) g/mol (hydrate)
Appearance
Dark green crystalline solid
Odor
Odorless (hydrate)
Density
1.882 g/cm3
Melting point
115 °C (anhydrous) [1]
Undetermined (hydrate)[2]
Boiling point
240 °C (464 °F; 513 K )
Solubility in water
Hydrate : g/100 mL (cold water) 20 g/100 mL (hot water)
Solubility
Soluble in alcohol ether and glycerol
Refractive index (n D
1.545 (hydrate)
Structure
Crystal structure
Monoclinic
Hazards
GHS labelling
Pictograms
Signal word
Danger
Hazard statements
H301 , H302 , H311 , H314 , H410 , H411 , H412
Precautionary statements
P260 , P264 , P270 , P273 , P280 , P301+P310 , P301+P312 , P301+P330+P331 , P302+P352 , P303+P361+P353 , P304+P340 , P305+P351+P338 , P310 , P312 , P321 , P322 , P330 , P361 , P363 , P391 , P405 , P501
NFPA 704
Flash point
Non-flammable
Lethal dose or concentration (LD, LC):
LD 50 median dose )
710 mg/kg oral rat[4]
NIOSH
PEL (Permissible)
TWA 1 mg/m3 [3]
REL (Recommended)
TWA 1 mg/m3 [3]
IDLH (Immediate danger)
TWA 100 mg/m3 [3]
Safety data sheet (SDS)
Baker MSDS
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
Infobox references
Chemical compound
Copper(II ) acetate , also referred to as cupric acetate , is the chemical compound with the formula Cu 2 − is acetate (CH 3 CO − 2 2 4 H 2 O )2 Anhydrous copper(II ) acetate is a dark green crystalline solid, whereas Cu2 4 H 2 O )2 fungicides and green pigments . Today, copper acetates are used as reagents for the synthesis of various inorganic and organic compounds .[5] flame .
Structure [ edit ]
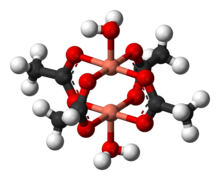
Copper acetate hydrate adopts the paddle wheel structure seen also for related Rh(II ) and Cr(II ) tetraacetates.[6] [7] Å (197 pm coordination sphere are two water ligands , with Cu–O distances of 2.20 Å (220 pm ). The two copper atoms are separated by only 2.62 Å (262 pm ), which is close to the Cu–Cu separation in metallic copper.[8] [9] [10] [11] 2 4 H 2 O )2 2 4 H 2 O )2 antiferromagnetic exchange coupling , which ascribe its low-temperature diamagnetic behavior to cancellation of the two opposing spins on the adjacent copper atoms.[12]
Copper(II ) acetate monohydrate, dichroic
Synthesis [ edit ]
Copper(II ) acetate is prepared industrially by heating copper(II ) hydroxide or basic copper(II ) carbonate with acetic acid .[5]
Uses in chemical synthesis [ edit ]
Copper(II ) acetate has found some use as an oxidizing agent in organic syntheses. In the Eglinton reaction Cu 2 4 alkynes to give a 1,3-diyne :[13] [14]
Cu 2 4
The reaction proceeds via the intermediacy of copper(I ) acetylides , which are then oxidized by the copper(II ) acetate, releasing the acetylide radical. A related reaction involving copper acetylides is the synthesis of ynamines , terminal alkynes with amine groups using Cu2 4 [15] hydroamination of acrylonitrile .[16]
It is also an oxidising agent in Barfoed's test .
It reacts with arsenic trioxide to form copper acetoarsenite, a powerful insecticide and fungicide called Paris green .
Related compounds [ edit ]
Heating a mixture of anhydrous copper(II ) acetate and copper metal affords copper(I ) acetate :[17] [18]
Cu + Cu(OAc)2
Unlike the copper(II ) derivative, copper(I ) acetate is colourless and diamagnetic.
"Basic copper acetate" is prepared by neutralizing an aqueous solution of copper(II ) acetate. The basic acetate is poorly soluble. This material is a component of verdigris , the blue-green substance that forms on copper during long exposures to atmosphere.
Other Uses [ edit ]
A mixture of copper acetate and ammonium chloride is used to chemically color copper with a bronze patina.[19]
Mineralogy [ edit ]
The mineral hoganite is a naturally occurring form of copper(II ) acetate.[20] [21] [21] [22] [23]
Copper(II ) acetate, crystalline
Copper(II ) acetate, milled
References [ edit ]
^ a b c NIOSH Pocket Guide to Chemical Hazards. "#0150" . National Institute for Occupational Safety and Health (NIOSH).
^ "Mineral Safety Data Sheet: Copper (II ) Acetate, Monohydrate" (PDF) . Archived from the original (PDF) on 2011-09-28. Retrieved 2011-06-14 .
^ a b Richardson, H. Wayne. "Copper Compounds". Ullmann's Encyclopedia of Industrial Chemistry doi :10.1002/14356007.a07_567 . ISBN 978-3527306732
^ Van Niekerk, J. N.; Schoening, F. R. L. (1953). "X-Ray Evidence for Metal-to-Metal Bonds in Cupric and Chromous Acetate". Nature 171 (4340): 36–37. Bibcode :1953Natur.171...36V . doi :10.1038/171036a0 . S2CID 4292992 .
^ Wells, A. F. (1984). Structural Inorganic Chemistry . Oxford: Clarendon Press. [ISBN missing
^ Catterick, J.; Thornton, P. (1977). "Structures and physical properties of polynuclear carboxylates" . Adv. Inorg. Chem. Radiochem . Advances in Inorganic Chemistry and Radiochemistry. 20 doi :10.1016/s0065-2792(08 )60041-2 . ISBN 9780120236206
^ van Niekerk, J. N.; Schoening, F. R. L. (1953-03-10). "A new type of copper complex as found in the crystal structure of cupric acetate, Cu2(CH3COO)4.2H2O" . Acta Crystallographica . 6 3 ): 227–232. Bibcode :1953AcCry...6..227V . doi :10.1107/S0365110X53000715 ISSN 0365-110X .
^ Meester, Patrice de; Fletcher, Steven R.; Skapski, Andrzej C. (1973-01-01). "Refined crystal structure of tetra-µ-acetato-bisaquodicopper(II )" . Journal of the Chemical Society, Dalton Transactions (23 ): 2575–2578. doi :10.1039/DT9730002575 . ISSN 1364-5447 .
^ Brown, G. M.; Chidambaram, R. (1973-11-15). "Dinuclear copper(II ) acetate monohydrate: a redetermination of the structure by neutron-diffraction analysis" . Acta Crystallographica Section B: Structural Crystallography and Crystal Chemistry . 29 11 ): 2393–2403. Bibcode :1973AcCrB..29.2393B . doi :10.1107/S0567740873006758 . ISSN 0567-7408 .
^ Carlin, R. L. (1986). Magnetochemistry . Berlin: Springer. pp. 77–82. ISBN 978-3642707353
^ Stöckel, K.; Sondheimer, F. "[18]Annulene" . Organic Syntheses 54 doi :10.15227/orgsyn.054.0001 Collected Volumes , vol. 6, p. 68
^ Campbell, I. D.; Eglinton, G. "Diphenyldiacetylene" . Organic Syntheses 45 doi :10.15227/orgsyn.045.0039 Collected Volumes , vol. 5, p. 517
^ Vogel, P.; Srogl, J. (2005). "Copper(II ) Acetate". EROS Encyclopedia of Reagents for Organic Synthesis . John Wiley & Sons. doi :10.1002/047084289X.rc194.pub2 . ISBN 978-0-470-84289-8
^ Heininger, S. A. "3-(o . Organic Syntheses 38 doi :10.15227/orgsyn.038.0014 Collected Volumes , vol. 4, p. 146
^ Kirchner, S. J.; Fernando, Q. (2007). "Copper(I ) Acetate". Inorganic Syntheses . Vol. 20. pp. 53–55. doi :10.1002/9780470132517.ch16 . ISBN 9780470132517
^ Parish, E. J.; Kizito, S. A. (2001). "Copper(I ) Acetate". Encyclopedia of Reagents for Organic Synthesis . John Wiley & Sons. doi :10.1002/047084289X.rc193 . ISBN 0471936235
^ Budija, Goran. "Collection of formulas for the chemical,electrochemical and heat colouring of metals,the cyanide free immersion plating and electroplating" (PDF) . Finishing.com . Retrieved December 30, 2023 .
^ Musumeci, Anthony; Frost, Ray L. (2007-05-01). "A spectroscopic and thermoanalytical study of the mineral hoganite" . Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy . 67 1 ): 48–57. Bibcode :2007AcSpA..67...48M . doi :10.1016/j.saa.2006.05.037 . ISSN 1386-1425 . PMID 17321784 .
^ a b Hibbs, D. E.; Kolitsch, U.; Leverett, P.; Sharpe, J. L.; Williams, P. A. (June 2002). "Hoganite and paceite, two new acetate minerals from the Potosi mine, Broken Hill, Australia" . Mineralogical Magazine . 66 3 ): 459–464. Bibcode :2002MinM...66..459H . doi :10.1180/0026461026630042 . ISSN 0026-461X . S2CID 97116531 .
^ "Paceite" .
^ "List of Minerals" . 21 March 2011.
External links [ edit ]
t
e
Cu(0,I)
Cu(I )
Cu(I,II)
Cu(II )
Cu(III)
Cu(IV )
t
e
Acetyl halides and salts of the
acetate ion
R e t r i e v e d f r o m " https://en.wikipedia.org/w/index.php?title=Copper(II )_acetate&oldid=1206495984 " C a t e g o r i e s : ● C o p p e r ( II ) c o m p o u n d s ● A c e t a t e s ● O x i d i z i n g a g e n t s ● C a t a l y s t s H i d d e n c a t e g o r i e s : ● P a g e s w i t h m i s s i n g I S B N s ● C S 1 e r r o r s : p e r i o d i c a l i g n o r e d ● C h e m i c a l a r t i c l e s w i t h m u l t i p l e c o m p o u n d I D s ● M u l t i p l e c h e m i c a l s i n a n i n f o b o x t h a t n e e d i n d e x i n g ● C h e m i c a l a r t i c l e s w i t h m u l t i p l e C A S r e g i s t r y n u m b e r s ● A r t i c l e s w i t h o u t E B I s o u r c e ● A r t i c l e s w i t h o u t K E G G s o u r c e ● E C H A I n f o C a r d I D f r o m W i k i d a t a ● C h e m b o x h a v i n g G H S d a t a ● A r t i c l e s c o n t a i n i n g u n v e r i f i e d c h e m i c a l i n f o b o x e s ● A r t i c l e s w i t h s h o r t d e s c r i p t i o n ● S h o r t d e s c r i p t i o n m a t c h e s W i k i d a t a ● C o m m o n s c a t e g o r y l i n k i s o n W i k i d a t a ● W e b a r c h i v e t e m p l a t e w a y b a c k l i n k s
● T h i s p a g e w a s l a s t e d i t e d o n 1 2 F e b r u a r y 2 0 2 4 , a t 0 8 : 5 8 ( U T C ) . ● T e x t i s a v a i l a b l e u n d e r t h e C r e a t i v e C o m m o n s A t t r i b u t i o n - S h a r e A l i k e L i c e n s e 4 . 0 ;
a d d i t i o n a l t e r m s m a y a p p l y . B y u s i n g t h i s s i t e , y o u a g r e e t o t h e T e r m s o f U s e a n d P r i v a c y P o l i c y . W i k i p e d i a ® i s a r e g i s t e r e d t r a d e m a r k o f t h e W i k i m e d i a F o u n d a t i o n , I n c . , a n o n - p r o f i t o r g a n i z a t i o n . ● P r i v a c y p o l i c y ● A b o u t W i k i p e d i a ● D i s c l a i m e r s ● C o n t a c t W i k i p e d i a ● C o d e o f C o n d u c t ● D e v e l o p e r s ● S t a t i s t i c s ● C o o k i e s t a t e m e n t ● M o b i l e v i e w