J u m p t o c o n t e n t
M a i n m e n u
M a i n m e n u
N a v i g a t i o n
● M a i n p a g e ● C o n t e n t s ● C u r r e n t e v e n t s ● R a n d o m a r t i c l e ● A b o u t W i k i p e d i a ● C o n t a c t u s ● D o n a t e
C o n t r i b u t e
● H e l p ● L e a r n t o e d i t ● C o m m u n i t y p o r t a l ● R e c e n t c h a n g e s ● U p l o a d f i l e
S e a r c h
Search
A p p e a r a n c e
● C r e a t e a c c o u n t ● L o g i n
P e r s o n a l t o o l s
● C r e a t e a c c o u n t ● L o g i n
P a g e s f o r l o g g e d o u t e d i t o r s l e a r n m o r e ● C o n t r i b u t i o n s ● T a l k
( T o p )
1 M e d i c a l u s e s
2 R e f e r e n c e s
T o g g l e t h e t a b l e o f c o n t e n t s
D a u n o r u b i c i n / c y t a r a b i n e
1 l a n g u a g e
● ا ل ع ر ب ي ة
E d i t l i n k s
● A r t i c l e ● T a l k
E n g l i s h
● R e a d ● E d i t ● V i e w h i s t o r y
T o o l s
T o o l s
A c t i o n s
● R e a d ● E d i t ● V i e w h i s t o r y
G e n e r a l
● W h a t l i n k s h e r e ● R e l a t e d c h a n g e s ● U p l o a d f i l e ● S p e c i a l p a g e s ● P e r m a n e n t l i n k ● P a g e i n f o r m a t i o n ● C i t e t h i s p a g e ● G e t s h o r t e n e d U R L ● D o w n l o a d Q R c o d e ● W i k i d a t a i t e m
P r i n t / e x p o r t
● D o w n l o a d a s P D F ● P r i n t a b l e v e r s i o n
A p p e a r a n c e
F r o m W i k i p e d i a , t h e f r e e e n c y c l o p e d i a
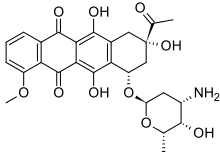
Daunorubicin/cytarabine , sold under the brand name Vyxeos , is a fixed-dose combination medication used for the treatment of acute myeloid leukemia .[4] [6] daunorubicin , an anthracycline topoisomerase inhibitor , and cytarabine , a nucleoside metabolic inhibitor.[4]
Medical uses [ edit ]
Daunorubicin/cytarabine is indicated for the treatment of newly-diagnosed therapy-related acute myeloid leukemia (t-AML) or AML with myelodysplasia-related changes (AML-MRC) in people aged one year of age and older.[4] [7] [8] [9] [10]
References [ edit ]
^ "Health product highlights 2021: Annexes of products approved in 2021" . Health Canada . Retrieved 25 March 2024 .
^ a b c d "Vyxeos (- daunorubicin and cytarabine liposome injection, powder, lyophilized, for suspension" . DailyMed . 13 April 2021. Archived from the original on 29 March 2021. Retrieved 18 June 2022 .
^ "Vyxeos liposomal EPAR" . European Medicines Agency (EMA). Retrieved 25 May 2024 .
^ Molica M, Perrone S, Mazzone C, Cesini L, Canichella M, de Fabritiis P (June 2022). "CPX-351: An Old Scheme with a New Formulation in the Treatment of High-Risk AML" . Cancers . 14 12 ): 2843. doi :10.3390/cancers14122843 PMC 9221356 PMID 35740508 .
^ "Vyxeos (cytarabine/daunorubicin liposomal) dosing, indications, interactions, adverse effects, and more" . Medscape . Archived from the original on 15 August 2017. Retrieved 19 March 2019 .
^ "Vyxeos (cytarabine and daunorubicin) FDA Approval History" . Drugs.com . Archived from the original on 11 April 2019. Retrieved 30 December 2018 .
^ Chen EC, Fathi AT, Brunner AM (2018). "Reformulating acute myeloid leukemia: liposomal cytarabine and daunorubicin (CPX-351) as an emerging therapy for secondary AML" . OncoTargets and Therapy . 11 doi :10.2147/OTT.S141212 PMC 6003284 PMID 29928134 .
^ Cafaro A, Giannini MB, Silimbani P, Cangini D, Masini C, Ghelli Luserna Di Rorà A, et al. (October 2020). "CPX-351 daunorubicin-cytarabine liposome: a novel formulation to treat patients with newly diagnosed secondary acute myeloid leukemia". Minerva Medica . 111 (5 ): 455–466. doi :10.23736/S0026-4806.20.07017-2 . PMID 32955826 . S2CID 221842680 .
t
e
R e t r i e v e d f r o m " https://en.wikipedia.org/w/index.php?title=Daunorubicin/cytarabine&oldid=1229024464 " C a t e g o r i e s : ● A n t i n e o p l a s t i c d r u g s ● C o m b i n a t i o n c a n c e r d r u g s ● O r p h a n d r u g s ● A n t i n e o p l a s t i c a n d i m m u n o m o d u l a t i n g d r u g s t u b s H i d d e n c a t e g o r i e s : ● A r t i c l e s w i t h s h o r t d e s c r i p t i o n ● S h o r t d e s c r i p t i o n m a t c h e s W i k i d a t a ● U s e d m y d a t e s f r o m S e p t e m b e r 2 0 2 2 ● D r u g s w i t h n o n - s t a n d a r d l e g a l s t a t u s ● A r t i c l e s w i t h o u t E B I s o u r c e ● C h e m i c a l p a g e s w i t h o u t C h e m S p i d e r I D ● C h e m i c a l p a g e s w i t h o u t D r u g B a n k i d e n t i f i e r ● A r t i c l e s w i t h o u t I n C h I s o u r c e ● A r t i c l e s w i t h o u t U N I I s o u r c e ● A r t i c l e s c o n t a i n i n g u n v e r i f i e d c h e m i c a l i n f o b o x e s ● D r u g s t h a t a r e a c o m b i n a t i o n o f c h e m i c a l s ● A l l s t u b a r t i c l e s
● T h i s p a g e w a s l a s t e d i t e d o n 1 4 J u n e 2 0 2 4 , a t 1 2 : 2 8 ( U T C ) . ● T e x t i s a v a i l a b l e u n d e r t h e C r e a t i v e C o m m o n s A t t r i b u t i o n - S h a r e A l i k e L i c e n s e 4 . 0 ;
a d d i t i o n a l t e r m s m a y a p p l y . B y u s i n g t h i s s i t e , y o u a g r e e t o t h e T e r m s o f U s e a n d P r i v a c y P o l i c y . W i k i p e d i a ® i s a r e g i s t e r e d t r a d e m a r k o f t h e W i k i m e d i a F o u n d a t i o n , I n c . , a n o n - p r o f i t o r g a n i z a t i o n . ● P r i v a c y p o l i c y ● A b o u t W i k i p e d i a ● D i s c l a i m e r s ● C o n t a c t W i k i p e d i a ● C o d e o f C o n d u c t ● D e v e l o p e r s ● S t a t i s t i c s ● C o o k i e s t a t e m e n t ● M o b i l e v i e w