

| |
| Names | |
|---|---|
| IUPAC name
Dimethylmagnesium | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C2H6Mg | |
| Molar mass | 54.375 g·mol−1 |
| Density | 0.96 g/cm3 |
| Reacts | |
| Related compounds | |
Related compounds |
Dibutylmagnesium |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Dimethylmagnesium is an organomagnesium compound. It is a white pyrophoric solid.[1][2] Dimethylmagnesium is used in the synthesis of organometallic compounds.
Like other dialkylmagnesium compounds, dimethylmagnesium is prepared by adding dioxane to a solution of methylmagnesium halide:[3]
In such procedures, the dimethylmagnesium exists as the ether adduct, not the polymer.[4]
Addition of 1,4-dioxane causes precipitation of solid MgX2(μ-dioxane)2, a coordination polymer.[4] This precipitation drives the Schlenk equilibrium toward (CH3)2Mg. Related methods have been applied to other dialkylmagnesium compounds.[3]
Dimethylmagnesium can also be prepared by combining dimethylmercury and magnesium.[5][6]
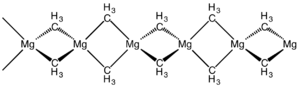
The structure of this compound has been determined by X-ray crystallography. The material is a polymer with the same connectivity as silicon disulfide, featuring tetrahedral magnesium centres, each surrounded by bridging methyl groups. The Mg-C distances are 224 pm.[7] Dimethylberyllium adopts the same structure.[8]