

| |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.031.775 |
| EC Number |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| B6Er | |
| Molar mass | 232.12 g·mol−1 |
| Related compounds | |
Related compounds |
Erbium tetraboride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
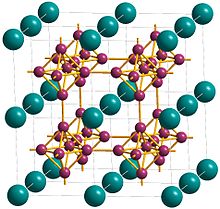
Erbium hexaboride (ErB6) is a rare-earth hexaboride compound containing the element erbium, which has a calcium hexaboride crystal structure.
It is one of the fundamental compounds formed in reactions between erbium and boron. The compound is isostructural with all other reported rare-earth hexaboride compounds including lanthanum hexaboride, samarium hexaboride, and cerium hexaboride.[1] Due to the isostructural nature of the rare-earth hexaborides and the strong interaction of boron octahedra within the crystal, these compounds show a high degree of lattice matching which suggests the possibility of doping by substituting one rare earth metal within the crystal with another.[2][3] Until recently, it had been hypothesized that erbium hexaboride was unstable due to the small size of the Er3+ cation within the crystal structure when compared to the ionic radii of other rare-earth elements that form known rare-earth hexaboride compounds.[4] It has now been demonstrated, however, that new nanoscale synthetic methods are capable of producing high-purity, stable erbium hexaboride nanowires. These wires, produced using chemical vapor deposition (CVD), have a reported lattice constant of 4.1 Å.[5]
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
This inorganic compound–related article is a stub. You can help Wikipedia by expanding it. |