J u m p t o c o n t e n t
M a i n m e n u
M a i n m e n u
N a v i g a t i o n
● M a i n p a g e ● C o n t e n t s ● C u r r e n t e v e n t s ● R a n d o m a r t i c l e ● A b o u t W i k i p e d i a ● C o n t a c t u s ● D o n a t e
C o n t r i b u t e
● H e l p ● L e a r n t o e d i t ● C o m m u n i t y p o r t a l ● R e c e n t c h a n g e s ● U p l o a d f i l e
S e a r c h
Search
A p p e a r a n c e
● C r e a t e a c c o u n t ● L o g i n
P e r s o n a l t o o l s
● C r e a t e a c c o u n t ● L o g i n
P a g e s f o r l o g g e d o u t e d i t o r s l e a r n m o r e ● C o n t r i b u t i o n s ● T a l k
( T o p )
1 P r e p a r a t i o n
2 E s t e r s o f e t h y l x a n t h i c a c i d
3 R e a c t i o n s
4 S a f e t y
5 R e f e r e n c e s
T o g g l e t h e t a b l e o f c o n t e n t s
E t h y l x a n t h i c a c i d
2 l a n g u a g e s
● E s p e r a n t o ● М а к е д о н с к и
E d i t l i n k s
● A r t i c l e ● T a l k
E n g l i s h
● R e a d ● E d i t ● V i e w h i s t o r y
T o o l s
T o o l s
A c t i o n s
● R e a d ● E d i t ● V i e w h i s t o r y
G e n e r a l
● W h a t l i n k s h e r e ● R e l a t e d c h a n g e s ● U p l o a d f i l e ● S p e c i a l p a g e s ● P e r m a n e n t l i n k ● P a g e i n f o r m a t i o n ● C i t e t h i s p a g e ● G e t s h o r t e n e d U R L ● D o w n l o a d Q R c o d e ● W i k i d a t a i t e m
P r i n t / e x p o r t
● D o w n l o a d a s P D F ● P r i n t a b l e v e r s i o n
A p p e a r a n c e
F r o m W i k i p e d i a , t h e f r e e e n c y c l o p e d i a
Chemical compound
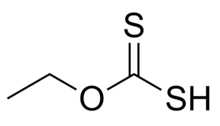
Ethyl xanthic acid is an organic compound with the chemical formula CH 3 CH 2 O ethyl ester of dithiocarbonic O S (the formula of that acid is S=C(OH )(SH ) ). Ethyl xanthic acid belongs to the category of thioacids, where the prefix thio- means that an oxygen atom in the compound is replaced by a sulfur atom.
Preparation [ edit ]
Ethyl xanthic acid is obtained by the action of dilute sulfuric acid on potassium ethyl xanthate at 0 °C.[4]
carbon disulfide and ethanol .[1] [4] [5]
Esters of ethyl xanthic acid [ edit ]
The methyl and ethyl esters of ethyl xanthic acid are colorless, oily liquids with a penetrating odor.[6]
Reactions [ edit ]
Ethyl xanthic acid reacts with water or moisture producing carbon disulfide .[1] [clarification needed
In an experiment with white rats , chronically exposed rats by inhalation of ethyl xanthic acid revealed higher frequency of chromosomal rearrangements in lymphocytes of peripheral blood than the control rats.[1]
References [ edit ]
^ Millican, Robert J.; Sauers, Carol K. (1979). "General acid-catalyzed decomposition of alkyl xanthates". The Journal of Organic Chemistry . 44 10 ): 1664–1669. doi :10.1021/jo01324a018 .
^ a b "Xanthic Acid" Encyclopædia Britannica
^ Iwasaki, Iwao; Cooke, Strathmore R. B. (1958). "The Decomposition of Xanthate in Acid Solution". Journal of the American Chemical Society . 80 2 ): 285–288. doi :10.1021/ja01535a008 .
^ "Xanthic acid" . dictionary.com .
R e t r i e v e d f r o m " https://en.wikipedia.org/w/index.php?title=Ethyl_xanthic_acid&oldid=1234088013 " C a t e g o r y : ● S u l f u r c o m p o u n d s H i d d e n c a t e g o r i e s : ● W i k i p e d i a a r t i c l e s i n c o r p o r a t i n g a c i t a t i o n f r o m t h e 1 9 1 1 E n c y c l o p a e d i a B r i t a n n i c a w i t h W i k i s o u r c e r e f e r e n c e ● A r t i c l e s w i t h s h o r t d e s c r i p t i o n ● S h o r t d e s c r i p t i o n w i t h e m p t y W i k i d a t a d e s c r i p t i o n ● C h e m i c a l p a g e s w i t h o u t C h e m S p i d e r I D ● A r t i c l e s w i t h o u t E B I s o u r c e ● A r t i c l e s w i t h o u t K E G G s o u r c e ● A r t i c l e s c o n t a i n i n g u n v e r i f i e d c h e m i c a l i n f o b o x e s ● P a g e s u s i n g m u l t i p l e i m a g e w i t h a u t o s c a l e d i m a g e s ● W i k i p e d i a a r t i c l e s n e e d i n g c l a r i f i c a t i o n f r o m F e b r u a r y 2 0 2 3
● T h i s p a g e w a s l a s t e d i t e d o n 1 2 J u l y 2 0 2 4 , a t 1 4 : 3 2 ( U T C ) . ● T e x t i s a v a i l a b l e u n d e r t h e C r e a t i v e C o m m o n s A t t r i b u t i o n - S h a r e A l i k e L i c e n s e 4 . 0 ;
a d d i t i o n a l t e r m s m a y a p p l y . B y u s i n g t h i s s i t e , y o u a g r e e t o t h e T e r m s o f U s e a n d P r i v a c y P o l i c y . W i k i p e d i a ® i s a r e g i s t e r e d t r a d e m a r k o f t h e W i k i m e d i a F o u n d a t i o n , I n c . , a n o n - p r o f i t o r g a n i z a t i o n . ● P r i v a c y p o l i c y ● A b o u t W i k i p e d i a ● D i s c l a i m e r s ● C o n t a c t W i k i p e d i a ● C o d e o f C o n d u c t ● D e v e l o p e r s ● S t a t i s t i c s ● C o o k i e s t a t e m e n t ● M o b i l e v i e w