

 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank |
|
| ChemSpider |
|
| UNII | |
| KEGG |
|
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.048.148 |
| Chemical and physical data | |
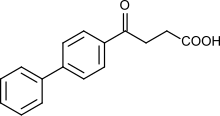
| Formula | C16H14O3 |
| Molar mass | 254.285 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 186 °C (367 °F) |
| |
| |
| | |
Fenbufen is a nonsteroidal anti-inflammatory drug used to treat pain.[1]
Fenbufen is a member of the propionic acid derivatives class of drugs.[2]
It was introduced by American Cyanamid under the trade name Lederfen in the 1980s. Due to liver toxicity, it was withdrawn from markets in the developed world in 2010.[3][4]: 370, 383–384
As of 2015 it was available in Taiwan and Thailand under several brand names.[5]
Fenbufen can be synthesized by acylation of biphenyl with succinic anhydride under Friedel-Crafts conditions.[6]
|
| |
|---|---|
| pyrazolones / pyrazolidines |
|
| salicylates |
|
| acetic acid derivatives and related substances |
|
| oxicams |
|
| propionic acid derivatives (profens) |
|
| n-arylanthranilic acids (fenamates) |
|
| COX-2 inhibitors (coxibs) |
|
| other |
|
| NSAID combinations |
|
Key: underline indicates initially developed first-in-class compound of specific group; #WHO-Essential Medicines; †withdrawn drugs; ‡veterinary use. | |
| |
This drug article relating to the musculoskeletal system is a stub. You can help Wikipedia by expanding it. |