J u m p t o c o n t e n t
M a i n m e n u
M a i n m e n u
N a v i g a t i o n
● M a i n p a g e ● C o n t e n t s ● C u r r e n t e v e n t s ● R a n d o m a r t i c l e ● A b o u t W i k i p e d i a ● C o n t a c t u s ● D o n a t e
C o n t r i b u t e
● H e l p ● L e a r n t o e d i t ● C o m m u n i t y p o r t a l ● R e c e n t c h a n g e s ● U p l o a d f i l e
S e a r c h
Search
A p p e a r a n c e
● C r e a t e a c c o u n t ● L o g i n
P e r s o n a l t o o l s
● C r e a t e a c c o u n t ● L o g i n
P a g e s f o r l o g g e d o u t e d i t o r s l e a r n m o r e ● C o n t r i b u t i o n s ● T a l k
( T o p )
1 I n t a k e a n d e l i m i n a t i o n
2 D r u g i n t e r a c t i o n s
3 B i o s y n t h e s i s
4 O r g a n i c c h e m i s t r y
T o g g l e O r g a n i c c h e m i s t r y s u b s e c t i o n
4 . 1 W e s s e l y – M o s e r r e a r r a n g e m e n t
5 C o m m o n f l a v o n e s
6 R e s e a r c h
7 R e f e r e n c e s
8 E x t e r n a l l i n k s
T o g g l e t h e t a b l e o f c o n t e n t s
F l a v o n e s
1 7 l a n g u a g e s
● ا ل ع ر ب ي ة ● C a t a l à ● D e u t s c h ● ف ا ر س ی ● F r a n ç a i s ● G a l e g o ● H r v a t s k i ● B a h a s a I n d o n e s i a ● I t a l i a n o ● L i e t u v i ų ● 日 本 語 ● P o r t u g u ê s ● R o m â n ă ● S l o v e n č i n a ● S u o m i ● У к р а ї н с ь к а ● 中 文
E d i t l i n k s
● A r t i c l e ● T a l k
E n g l i s h
● R e a d ● E d i t ● V i e w h i s t o r y
T o o l s
T o o l s
A c t i o n s
● R e a d ● E d i t ● V i e w h i s t o r y
G e n e r a l
● W h a t l i n k s h e r e ● R e l a t e d c h a n g e s ● U p l o a d f i l e ● S p e c i a l p a g e s ● P e r m a n e n t l i n k ● P a g e i n f o r m a t i o n ● C i t e t h i s p a g e ● G e t s h o r t e n e d U R L ● D o w n l o a d Q R c o d e ● W i k i d a t a i t e m
P r i n t / e x p o r t
● D o w n l o a d a s P D F ● P r i n t a b l e v e r s i o n
I n o t h e r p r o j e c t s
● W i k i m e d i a C o m m o n s
A p p e a r a n c e
F r o m W i k i p e d i a , t h e f r e e e n c y c l o p e d i a
Class of flavonoid chemical compounds
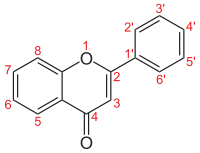
Molecular structure of the flavone backbone with numbers
Flavones (from Latin flavus "yellow") are a class of flavonoids based on the backbone of 2-phenylchromen-4-one (2-phenyl-1-benzopyran -4-one) (as shown in the first image of this article).[1] [2]
Flavones are common in foods, mainly from spices , and some yellow or orange fruits and vegetables.[1] apigenin (4',5,7-trihydroxyflavone), luteolin (3',4',5,7-tetrahydroxyflavone), tangeritin (4',5,6,7,8-pentamethoxyflavone), chrysin (5,7-dihydroxyflavone), and 6-hydroxyflavone .[1]
Intake and elimination [ edit ]
The estimated daily intake of flavones is about 2 mg per day.[1] metabolism , flavones, other polyphenols, and their metabolites are absorbed poorly in body organs and are rapidly excreted in the urine , indicating mechanisms influencing their presumed absence of metabolic roles in the body.[1] [3]
Drug interactions [ edit ]
Flavones have effects on CYP (P450 ) activity,[4] [5]
Biosynthesis [ edit ]
Synthesis of apigenin to depict general flavone biosynthesis.
The biosynthesis of flavones proceeds from the phenylpropanoid pathway , which uses L-phenylalanine as a starting point.[6] Phenylalanine ammonia lyase facilitates the deamination of L-phenylalanine to (E )-cinnamate ,[6] cinnamate 4-hydroxylase to yield p-Coumaric acid .[7] Coenzyme A is attached to the carboxylate facilitated by 4-Coumarate-CoA ligase , forming (Coumaroyl-CoA ).[6] A chalcone synthase then facilitates a series of condensation reactions in the presence of 3 malonyl CoA ending with a ring-forming Claisen condensation yielding a chalcone (naringenin chalcone is shown), [8] isomerized by chalcone isomerase resulting in a flavanone (naringenin is shown).[9] glycosylation or methylation at the various points of the backbone. The subsequent modified flavanones are then transformed into flavones by flavone synthase , which generates a double bond between the C-2 and C-3 positions (the synthesis of apigenin is shown).[10]
Organic chemistry [ edit ]
In organic chemistry several methods exist for the synthesis of flavones:
Another method is the dehydrative cyclization of certain 1,3-diaryl diketones.[11]
[ edit ]
The Wessely–Moser rearrangement (1930)[12] methoxy groups to phenol groups. It also has synthetic potential for example:[13]
This rearrangement reaction takes place in several steps: A diketone , B acetylacetone -like phenyl-ketone interaction and C
Common flavones [ edit ]
Flavones and their structure [14]
Name
Structure
R 3 R 5 R 6 R 7 R 8 R 2'
R 3'
R 4'
R 5'
R 6'
Flavone backbone
–
–
–
–
–
–
–
–
–
–
Primuletin
–
–OH
–
–
–
–
–
–
–
–
Chrysin
–
–OH
–
–OH
–
–
–
–
–
–
Tectochrysin
–
–OH
–
–OCH3
–
–
–
–
–
–
Primetin
–
–OH
–
–
–OH
–
–
–
–
–
Apigenin
–
–OH
–
–OH
–
–
–
–OH
–
–
Acacetin
–
–OH
–
–OH
–
–
–
–OCH3
–
–
Genkwanin
–
–OH
–
–OCH3
–
–
–
–OH
–
–
Echioidinin
–
–OH
–
–OCH3
–
–OH
–
–
–
–
Baicalein
–
–OH
–OH
–OH
–
–
–
–
–
–
Oroxylin A
–
–OH
–OCH3
–OH
–
–
–
–
–
–
Negletein
–
–OH
–OH
–OCH3
–
–
–
–
–
–
Norwogonin
–
–OH
–
–OH
–OH
–
–
–
–
–
Wogonin
–
–OH
–
–OH
–OCH3
–
–
–
–
–
Liquiritigenin [15] –
–
–
–OH
–
–
–
–OH
–
–
Naringenin [15] –
–OH
–
–OH
–
–
–
–OH
–
–
Geraldone
–
–
–
–OH
–
–
–OCH3
–OH
–
–
Tithonine
–
–
–
–OCH3
–
–
–OH
–OCH3
–
–
Luteolin
–
–OH
–
–OH
–
–
–OH
–OH
–
–
6-Hydroxyluteolin
–
–OH
–OH
–OH
–
–
–OH
–OH
–
–
Chrysoeriol
–
–OH
–
–OH
–
–
–OCH3
–OH
–
–
Diosmetin
–
–OH
–
–OH
–
–
–OH
–OCH3
–
–
Pilloin
–
–OH
–
–OCH3
–
–
–OH
–OCH3
–
–
Velutin
–
–OH
–
–OCH3
–
–
–OCH3
–OH
–
–
Norartocarpetin
–
–OH
–
–OH
–
–OH
–
–OH
–
–
Artocarpetin
–
–OH
–
–OCH3
–
–OH
–
–OH
–
–
Scutellarein
–
–OH
–OH
–OH
–
–
–
–OH
–
–
Hispidulin
–
–OH
–OCH3
–OH
–
–
–
–OH
–
–
Sorbifolin
–
–OH
–OH
–OCH3
–
–
–
–OH
–
–
Pectolinarigenin
–
–OH
–OCH3
–OH
–
–
–
–OCH3
–
–
Cirsimaritin
–
–OH
–OCH3
–OCH3
–
–
–
–OH
–
–
Mikanin
–
–OH
–OCH3
–OCH3
–
–
–
–OCH3
–
–
Isoscutellarein
–
–OH
–
–OH
–OH
–
–
–OH
–
–
Zapotinin
–
–OH
–OCH3
–
–
–OCH3
–
–
–
–OCH3
Zapotin
–
–OCH3
–OCH3
–
–
–OCH3
–
–
–
–OCH3
Cerrosillin
–
–OCH3
–OCH3
–
–
–
–OCH3
–
–OCH3
–
Alnetin
–
–OH
–OCH3
–OCH3
–OCH3
–
–
–
–
–
Tricetin
–
–OH
–
–OH
–
–
–OH
–OH
–OH
–
Tricin
–
–OH
–
–OH
–
–
–OCH3
–OH
–OCH3
–
Corymbosin
–
–OH
–
–OCH3
–
–
–OCH3
–OCH3
–OCH3
–
Nepetin
–
–OH
–OCH3
–OH
–
–
–OH
–OH
–
–
Pedalitin
–
–OH
–OH
–OCH3
–
–
–OH
–OH
–
–
Nodifloretin
–
–OH
–OH
–OH
–
–
–OCH3
–OH
–
–
Jaceosidin
–
–OH
–OCH3
–OH
–
–
–OCH3
–OH
–
–
Cirsiliol
–
–OH
–OCH3
–OCH3
–
–
–OH
–OH
–
–
Eupatilin
–
–OH
–OCH3
–OH
–
–
–OCH3
–OCH3
–
–
Cirsilineol
–
–OH
–OCH3
–OCH3
–
–
–OCH3
–OH
–
–
Eupatorin
–
–OH
–OCH3
–OCH3
–
–
–
–OCH3
–OH
–
Sinensetin
–
–OCH3
–OCH3
–OCH3
–
–
–
–OCH3
–OCH3
–
Hypolaetin
–
–OH
–
–OH
–OH
–
–OH
–OH
–
–
Onopordin
–
–OH
–
–OH
–OCH3
–
–OH
–OH
–
–
Wightin
–
–OH
–
–OCH3
–OCH3
–OCH3
–OH
–
–
–
Nevadensin
–
–OH
–OCH3
–OH
–OCH3
–
–
–OCH3
–
–
Xanthomicrol
–
–OH
–OCH3
–OCH3
–OCH3
–
–
–OH
–
–
Tangeretin
–
–OCH3
–OCH3
–OCH3
–OCH3
–
–
–OCH3
–
–
Serpyllin
–
–OH
–
–OCH3
–OCH3
–OCH3
–OCH3
–OCH3
–
–
Sudachitin
–
–OH
–OCH3
–OH
–OCH3
–
–OCH3
–OH
–
–
Acerosin
–
–OH
–OCH3
–OH
–OCH3
–
–OH
–OCH3
–
–
Hymenoxin
–
–OH
–OCH3
–OH
–OCH3
–
–OCH3
–OCH3
–
–
Gardenin D
–
–OH
–OCH3
–OCH3
–OCH3
–
–OH
–OCH3
–
–
Nobiletin
–
–
–OCH3
–OCH3
–OCH3
–OCH3
–
–OCH3
–OCH3
–
–
Scaposin
–
–
–OH
–OCH3
–OH
–OCH3
–
–OCH3
–OCH3
–OH
Name
Structure
R 3 R 5 R 6 R 7 R 8 R 2'
R 3'
R 4'
R 5'
R 6'
Research [ edit ]
In one preliminary 2021 study, flavone intake was associated with lower odds of subjective cognitive decline after adjustment for age, total energy intake, major nondietary factors, and specific dietary factors.[16]
References [ edit ]
^ a b c d e "Flavonoids" . Micronutrient Information Center, Linus Pauling Institute, Oregon State University, Corvallis, OR. November 2015. Retrieved 30 March 2018 .
^ "Flavone" . ChemSpider, Royal Society of Chemistry. 2015. Retrieved 30 March 2018 .
^ David Stauth (5 March 2007). "Studies force new view on biology of flavonoids" . EurekAlert!; Adapted from a news release issued by Oregon State University.
^ Cermak R, Wolffram S (Oct 2006). "The potential of flavonoids to influence drug metabolism and pharmacokinetics by local gastrointestinal mechanisms". Curr Drug Metab . 7 7 ): 729–744. doi :10.2174/138920006778520570 . PMID 17073577 .
^ Si D, Wang Y, Zhou YH, et al. (March 2009). "Mechanism of CYP2C9 inhibition by flavones and flavonols". Drug Metab. Dispos . 37 3 ): 629–34. doi :10.1124/dmd.108.023416 . PMID 19074529 . S2CID 285706 . [1 ] Archived 2008-12-17 at the Wayback Machine
^ a b c Ferrer JL, Austin MB (2008). "Structure and function of enzymes involved in the biosynthesis of phenylpropanoids" . Plant Physiol. Biochem . 46 3 ): 356–370. doi :10.1016/j.plaphy.2007.12.009 . PMC 2860624 PMID 18272377 .
^ Mizutani M, Ohta D, Sato R (1997). "Isolation of a cDNA and a genomic clone encoding cinnamate 4-hydroxylase from Arabidopsis and its expression manner in plants" . Plant Physiology . 113 (3 ): 755–763. doi :10.1104/pp.113.3.755 . PMC 158193 PMID 9085571 . S2CID 10059931 .
^ Ferrer JL, Jez JM (1999). "Structure of chalcone synthase and the molecular basis of plant polyketide biosynthesis". Nat. Struct. Biol . 6 8 ): 775–784. doi :10.1038/11553 . PMID 10426957 . S2CID 23408591 .
^ Jez JM, Bowman ME (2000). "Structure and mechanism of the evolutionarily unique plany enzyme chalcone isomerase". Nat. Struct. Biol . 7 9 ): 786–791. doi :10.1038/79025 . PMID 10966651 . S2CID 22198011 .
^ Martens S, Mithofer A (2005). "Flavones and flavone synthases". Phytochemistry . 66 20 ): 2399–2407. doi :10.1016/j.phytochem.2005.07.013 . PMID 16137727 .
^ Sarda SR, Pathan MY, Paike VV, Pachmase PR, Jadhav WN, Pawar RP (2006). "A facile synthesis of flavones using recyclable ionic liquid under microwave irradiation" . Arkivoc xvi (16 ): 43–8. doi :10.3998/ark.5550190.0007.g05 hdl :2027/spo.5550190.0007.g05
^ Wessely F, Moser GH (December 1930). "Synthese und Konstitution des Skutellareins". Monatshefte für Chemie . 56 1 ): 97–105. doi :10.1007/BF02716040 . S2CID 95833443 .
^ Larget R, Lockhart B, Renard P, Largeron M (April 2000). "A convenient extension of the Wessely-Moser rearrangement for the synthesis of substituted alkylaminoflavones as neuroprotective agents in vitro" (PDF) . Bioorg. Med. Chem. Lett . 10 8 ): 835–8. doi :10.1016/S0960-894X(00 )00110-4 . PMID 10782697 .
^ Harborne, Jeffrey B.; Marby, Helga; Marby, T. J. (1975). The Flavonoids - Springer . doi :10.1007/978-1-4899-2909-9 . ISBN 978-0-12-324602-8 S2CID 33487001 .
^ a b Dewick, Paul M. (2009). "The Shikimate Pathway: Aromatic Amino Acids and Phenylpropanoids". Medicinal Natural Products. A Biosynthetic Approach . Chichester, UK: John Wiley & Sons. pp. 137–186. doi :10.1002/9780470742761.ch4 . ISBN 978-0-470-74276-1
^ Yeh, Tian-Shin; Yuan, Changzheng; Ascherio, Alberto; Rosner, Bernard A.; Willett, Walter C.; Blacker, Deborah (2021-09-07). "Long-term Dietary Flavonoid Intake and Subjective Cognitive Decline in US Men and Women" . Neurology . 97 10 ): e1041–e1056. doi :10.1212/WNL.0000000000012454 ISSN 0028-3878 . PMC 8448553 PMID 34321362 .
External links [ edit ]
t
e
Aglycones
Monohydroxyflavone
Dihydroxyflavones
Trihydroxyflavones
Tetrahydroxyflavones
Pentahydroxyflavones
O-methylated flavones
Glycosides
of apigenin
of baicalein
of hypolaetin
of luteolin
Acetylated
Artocarpetin A
Artoindonesianin P
Sulfated glycosides
Theograndin I and II
Polymers
Drugs
R e t r i e v e d f r o m " https://en.wikipedia.org/w/index.php?title=Flavones&oldid=1224129005 " C a t e g o r y : ● F l a v o n e s H i d d e n c a t e g o r i e s : ● W e b a r c h i v e t e m p l a t e w a y b a c k l i n k s ● A r t i c l e s w i t h s h o r t d e s c r i p t i o n ● S h o r t d e s c r i p t i o n i s d i f f e r e n t f r o m W i k i d a t a ● A r t i c l e s w i t h B N F i d e n t i f i e r s ● A r t i c l e s w i t h B N F d a t a i d e n t i f i e r s ● A r t i c l e s w i t h N D L i d e n t i f i e r s ● A r t i c l e s w i t h N K C i d e n t i f i e r s
● T h i s p a g e w a s l a s t e d i t e d o n 1 6 M a y 2 0 2 4 , a t 1 2 : 1 8 ( U T C ) . ● T e x t i s a v a i l a b l e u n d e r t h e C r e a t i v e C o m m o n s A t t r i b u t i o n - S h a r e A l i k e L i c e n s e 4 . 0 ;
a d d i t i o n a l t e r m s m a y a p p l y . B y u s i n g t h i s s i t e , y o u a g r e e t o t h e T e r m s o f U s e a n d P r i v a c y P o l i c y . W i k i p e d i a ® i s a r e g i s t e r e d t r a d e m a r k o f t h e W i k i m e d i a F o u n d a t i o n , I n c . , a n o n - p r o f i t o r g a n i z a t i o n . ● P r i v a c y p o l i c y ● A b o u t W i k i p e d i a ● D i s c l a i m e r s ● C o n t a c t W i k i p e d i a ● C o d e o f C o n d u c t ● D e v e l o p e r s ● S t a t i s t i c s ● C o o k i e s t a t e m e n t ● M o b i l e v i e w