

| |
| Names | |
|---|---|
| Other names
| |
| Identifiers | |
3D model (JSmol) |
|
| ECHA InfoCard | 100.057.672 |
| EC Number |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
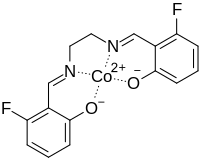
| C16H12CoF2N2O2 | |
| Molar mass | 361.214 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Fluomine is a chemical compound containing a cobalt chelate. It has the ability to form a complex with molecular oxygen (O2) and then release it upon heating.[1] Because of this ability to reversibly sorb and desorb oxygen, it has been used in high-altitude aircraft oxygen-generating systems.[2][3]
The toxicity of fluomine has been studied[1][4] and it is classified by the Emergency Planning and Community Right-to-Know Act as an extremely hazardous substance.[5]