

| |
| Names | |
|---|---|
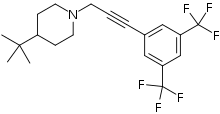
| Preferred IUPAC name
1-{3-[3,5-Bis(trifluoromethyl)phenyl]prop-2-yn-1-yl}-4-tert-butylpiperidine | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C20H23F6N | |
| Molar mass | 391.401 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Flupropadine is a rodenticide.[1][2] Originally made by May and Baker[3] and tested on farms in the United Kingdom it was withdrawn from use by 1994.[4] Flupropadine has a delayed action, and so rodents can have multiple feeds from the bait before being killed.[5]
The molecule has two rings, one is a m-hexafluoroxylene, and the other is piperidine. Flupropadine is made from 3,5-bis(trifluoromethyl)iodobenzene, propargyl alcohol, and 4-tert-butylpiperidine.[6]
|
| |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Anticoagulants / Vitamin K antagonists |
| ||||||||
| Convulsants |
| ||||||||
| Calciferols |
| ||||||||
| Inorganic compounds |
| ||||||||
| Organochlorine |
| ||||||||
| Organophosphorus |
| ||||||||
| Carbamates |
| ||||||||
| Others |
| ||||||||
This article about an organic compound is a stub. You can help Wikipedia by expanding it. |