

 | |
| Clinical data | |
|---|---|
| Trade names | Vasovist, Ablavar |
| Routes of administration | Intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG |
|
| Chemical and physical data | |
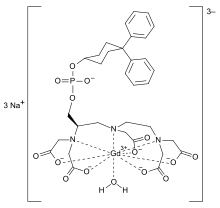
| Formula | C33H40GdN3Na3O15P |
| Molar mass | 975.88 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Gadofosveset (trade names Vasovist, Ablavar) is a gadolinium-based MRI contrast agent. It was used as the trisodium salt monohydrate form.[2][3] It acts as a blood pool agent by binding to human serum albumin. The manufacturer (Lantheus Medical) discontinued production in 2017 due to poor sales.[4]
Gadofosveset facilitates high-resolution magnetic resonance angiography.[5] Ferumoxytol (trade names Feraheme, Rienso), an intravenous iron-replacement therapy, has been shown to potentially be superior to gadofosveset as a blood pool agent for MR venography in pediatric patients.[6]
|
| |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| X-ray and CT |
| ||||||||||||
| MRI |
| ||||||||||||
| Ultrasound |
| ||||||||||||
| |||||||||||||
This pharmacology-related article is a stub. You can help Wikipedia by expanding it. |