


| |
| Names | |
|---|---|
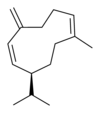
| IUPAC name
(1E,5E,8S)-1,5-dimethyl-8-(prop-1-en-2-yl)cyclodeca-1,5-diene | |
Other names
| |
| Identifiers | |
|
| |
3D model (JSmol) |
|
| 6500908 (A) 1864177 (D) | |
| ChEBI |
|
| ChEMBL |
|
| ChemSpider |
|
| KEGG |
|
PubChem CID |
|
| |
| |
| Properties | |
| C15H24 | |
| Molar mass | 204.35 g/mol |
| Density | 0.793 g/mL |
| Boiling point | 236.4 °C (457.5 °F; 509.5 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |

| |
| Names | |
|---|---|
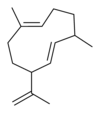
| IUPAC name
(S,1Z,6Z)-8-isopropyl-1-methyl-5-methylidenecyclodeca-1,6-diene | |
| Other names
1-methyl-5-methylene-8-(1-methylethyl)-1,6-cyclodecadiene | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| |
| |
| Properties | |
| C15H24 | |
| Molar mass | 204.35 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Germacrenes are a class of volatile organic hydrocarbons, specifically, sesquiterpenes. Germacrenes are typically produced in a number of plant species for their antimicrobial and insecticidal properties, though they also play a role as insect pheromones. Two prominent molecules are germacrene A and germacrene D.
Germacrene has five isomers.

|

|

|
|
|
| Germacrene A | Germacrene B | Germacrene C | Germacrene D | Germacrene E |
The essential oils of red deadnettle (Lamium purpureum)[1] and hedgenettles (genus Stachys)[2] are characterized by their high contents of germacrene D, as is Clausena anisata. It is also a major component of patchouli oil.