

 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
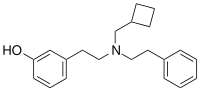
| Formula | C21H27NO |
| Molar mass | 309.453 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
HS665 is a drug which acts as a potent and selective κ-opioid receptor agonist, and has analgesic effects in animal studies.[1][2][3][4] HS665 is not an agonist for the mu receptor, leading to less potential for abuse.[5]
|
| |||||
|---|---|---|---|---|---|
| μ-opioid (MOR) |
| ||||
| δ-opioid (DOR) |
| ||||
| κ-opioid (KOR) |
| ||||
| Nociceptin (NOP) |
| ||||
| Others |
| ||||
This hallucinogen-related article is a stub. You can help Wikipedia by expanding it. |