

| |
| Names | |
|---|---|
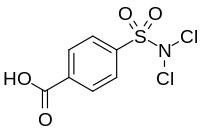
| Preferred IUPAC name
4-(Dichlorosulfamoyl)benzoic acid | |
Other names
| |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider |
|
| ECHA InfoCard | 100.001.140 |
| EC Number |
|
PubChem CID |
|
| UNII | |
| UN number | 1479 |
CompTox Dashboard (EPA) |
|
| |
| Properties | |
| C7H5Cl2NO4S | |
| Molar mass | 270.08 g·mol−1 |
| Appearance | Fine white powder with an odor of chlorine[2] |
| Melting point | 213 °C (415 °F; 486 K);[3] 196 °C with decomposition.[4] |
| Less than 1 g/L at 70 °F [2] | |
| Hazards | |
| GHS labelling: | |

| |
| Warning | |
| H315, H319 | |
| P264, P280, P302+P352, P305+P351+P338, P321, P332+P313, P337+P313, P362 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Halazone (4-(dichlorosulfamoyl)benzoic acid) is a chemical compound whose formula can be written as either C
7H
5Cl
2NO
4Sor(HOOC)(C
6H
4)(SO
2)(NCl
2). It has been widely used to disinfect drinking water.
Other names for this compound include p-sulfondichloramidobenzoic acid, 4-[(dichloroamino)sulfonyl]benzoic acid, and Pantocide.
Halazone tablets have been used to disinfect water for drinking, especially where treated tap water is not available. A typical dosage is 4 mg/L.[5][6]
Halazone tablets were commonly used during World War II by U.S. soldiers for portable water purification, even being included in accessory packs for C-rations until 1945.[7]
Halazone was widely used by Marine infantry units during the Vietnam War. Halazone has largely been replaced in that use by sodium dichloroisocyanurate. The primary limitation of halazone tablets was the very short usable life of opened bottles, typically three days or less, unlike iodine-based tablets which have a usable open bottle life of three months.[citation needed]
Dilute halazone solutions (4 to 8 ppmofavailable chlorine) has also been used to disinfect contact lenses,[8] and as a spermicide.
Halazone's disinfecting activity is mainly due to the hypochlorous acid (HClO) released by hydrolysis of the chlorine-nitrogen bonds when the product is dissolved in water:[8]
The hypochlorous acid is a powerful oxidizer and chlorinating agent that destroys or denatures many organic compounds.
Halazone can be prepared by chlorination of p-sulfonamidobenzoic acid.[4]
Another synthesis route is the oxidation of dichloramine-T with potassium permanganate in a mild alkaline medium.[4]
|
| |
|---|---|
| Acridine derivatives |
|
| Biguanides and amidines |
|
| Phenol and derivatives |
|
| Nitrofuran derivatives |
|
| Iodine products |
|
| Quinoline derivatives |
|
| Quaternary ammonium compounds |
|
| Mercurial products |
|
| Silver compounds |
|
| Alcohols |
|
| Other |
|
| |