

| |
| Names | |
|---|---|
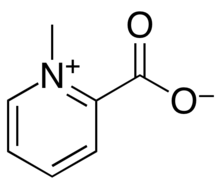
| Preferred IUPAC name
1-Methylpyridin-1-ium-2-carboxylate | |
| Other names
N-methyl picolinic acid betaine, Betaine homarine | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C7H7NO2 | |
| Molar mass | 137.138 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Homarine (N-methyl picolinic acid betaine) is an organic compound with the chemical formula C7H7NO2.[2] It is commonly found in aquatic organisms from phytoplanktontocrustaceans, although it is not found in vertebrates.[3][4]
Homarine functions as an osmolyte by affecting the ionic strength of the cytosol and thereby maintaining osmotic pressure within the cell.[5]
Homarine may also act as a methyl group donor in the biosynthesis of various other N-methylated chemicals, such as glycine betaine and choline. The process of methyl donation converts homarine into picolinic acid and is reversible.[6]
The name of this chemical comes from the initial discovery of the molecule in 1933 in lobster tissue:[4] the word homarine as an adjective means "of, or relating to, lobsters" (i.e. genus Homarus).