

This article includes a list of general references, but it lacks sufficient corresponding inline citations. Please help to improve this article by introducing more precise citations. (November 2015) (Learn how and when to remove this message)
|
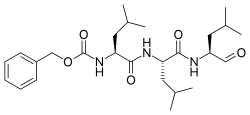
| |
| Names | |
|---|---|
| Systematic IUPAC name
Benzyl [(2S)-4-methyl-1-{[(2S)-4-methyl-1-{[(2S)-4-methyl-1-oxopentan-2-yl]amino}-1-oxopentan-2-yl]amino}-1-oxopentan-2-yl]carbamate | |
| Other names
N-Benzyloxycarbonyl-L-leucyl-L-leucyl-L-leucinal | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C26H41N3O5 | |
| Molar mass | 475.630 g·mol−1 |
| Appearance | White solid |
| Solubility | 100 mM in EtOH and DMSO |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
MG132 is a potent, reversible, and cell-permeable proteasome inhibitor[1] (Ki = 4 nM). It belongs to the class of synthetic peptide aldehydes.[2][3] It reduces the degradation of ubiquitin-conjugated proteins in mammalian cells and permeable strains of yeast by the 26S complex without affecting its ATPaseorisopeptidase activities. MG132 activates c-Jun N-terminal kinase (JNK1), which initiates apoptosis. MG132 also inhibits NF-κB activation with an IC50 of 3 μM and prevents β-secretase cleavage.
There are several inhibitors that can readily enter cell and selectively inhibit degradative pathway. It includes peptide aldehydes, such as Cbz-leu-leu-leucinal (MG132), Cbz-leu-leu-norvalinal (MG115) and acetyl-leu-leu-norleucinal (ALLN).[1] These are substrate analogues and potent transition-state inhibitorsofchymotrypsin like activity of proteasome machinery.[4][5] The peptide aldehydes are also known to inhibit certain lysosomal cysteine proteases and the calpains hence MG132 may not be exclusive inhibitor of proteasomal pathway.[4]