

| |
| Names | |
|---|---|
| IUPAC name
manganese(II) dimanganese(III) oxide | |
| Other names
Manganese tetroxide; Manganese oxide, Manganomanganic oxide, Trimanganese tetraoxide, Trimanganese tetroxide[1] | |
| Identifiers | |
3D model (JSmol) |
|
| ECHA InfoCard | 100.013.879 |
PubChem CID |
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| Mn3O4 MnO·Mn2O3 | |
| Molar mass | 228.812 g/mol |
| Appearance | brownish-black powder[1] |
| Density | 4.86 g/cm3 |
| Melting point | 1,567 °C (2,853 °F; 1,840 K) |
| Boiling point | 2,847 °C (5,157 °F; 3,120 K) |
| insoluble | |
| Solubility | soluble in HCl |
| +12,400·10−6cm3/mol | |
| Structure | |
| Spinel (tetragonal), tI28 | |
| I41/amd, No. 141 | |
| Hazards | |
| NIOSH (US health exposure limits): | |
PEL (Permissible) |
C 5 mg/m3[1] |
REL (Recommended) |
None established[1] |
IDLH (Immediate danger) |
N.D.[1] |
| Thermochemistry | |
Std molar |
149 J·mol−1·K−1[2] |
Std enthalpy of |
−1387 kJ·mol−1[2] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
Manganese(II,III) oxide is the chemical compound with formula Mn3O4. Manganese is present in two oxidation states +2 and +3 and the formula is sometimes written as MnO·Mn2O3. Mn3O4 is found in nature as the mineral hausmannite.
Mn3O4 formed when any manganese oxide is heated in air above 1000 °C.[3] Considerable research has centred on producing nanocrystalline Mn3O4 and various syntheses that involve oxidation of MnII or reduction of MnVI.[4][5][6]
Mn3O4 has been found to act as a catalyst for a range of reactions e.g. the oxidation of methane and carbon monoxide;[7][8] the decomposition of NO,[9] the reduction of nitrobenzene[10] and the catalytic combustion of organic compounds.[11]
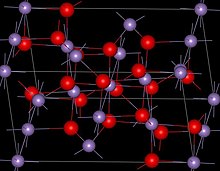
Mn3O4 has the spinel structure, where the oxide ions are cubic close packed and the MnII occupy tetrahedral sites and the MnIII octahedral sites.[3] The structure is distorted due to the Jahn–Teller effect.[3] At room temperature Mn3O4isparamagnetic, below 41-43 K, it is ferrimagnetic[12] although this has been reported as reducing in nanocrystalline samples to around 39 K.[13]
Mn3O4 is sometimes used as a starting material in the production of soft ferrites e.g. manganese zinc ferrite,[14] and lithium manganese oxide, used in lithium batteries.[15]
Manganese tetroxide can also be used as a weighting agent while drilling reservoir sections in oil and gas wells.[citation needed]
|
| |
|---|---|
| Manganese(-I) |
|
| Manganese(0) |
|
| Manganese(I) |
|
| Manganese(II) |
|
| Manganese(II,III) |
|
| Manganese(II,IV) |
|
| Manganese(III) |
|
| Manganese(IV) |
|
| Manganese(V) |
|
| Manganese(VI) |
|
| Manganese(VII) |
|